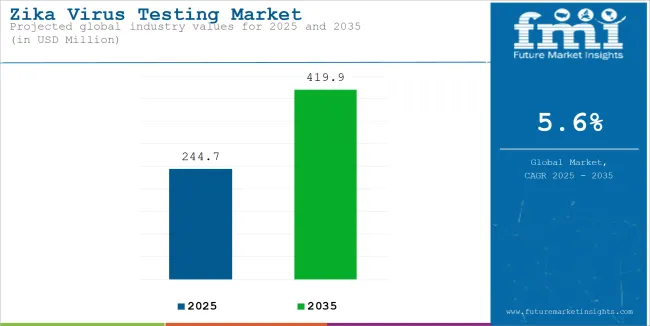
The global sales of Zika virus testing is estimated to be worth USD 244.7 million in 2025 and anticipated to reach a value of USD 419.9 million by 2035. Sales are projected to rise at a CAGR of 5.6% over the forecast period between 2025 and 2035. The revenue generated by Zika virus testing in 2024 was USD 232.7 million.
| Attributes | Key Insights |
|---|---|
| Historical Size, 2024 | USD 232.7 million |
| Estimated Size, 2025 | USD 244.7 million |
| Projected Size, 2035 | USD 419.9 million |
| CAGR (2025 to 2035) | 5.6% |
Zika virus is a virus transmitted primarily through mosquito bites as well as other means such as blood transfusion, sexual intercourse, and from mother to fetus during pregnancy. It usually has mild symptoms like fever, rash, and joint pains but can bring about severe conditions in some individuals.
The diagnosis of Zika virus includes molecular diagnostics such as PCR to detect viral RNA and serological tests including ELISA and rapid IgG/IgM for antibody detection. This market has been driven significantly by the increased demand for precise and swift diagnosis during an outbreak, ensuring timely intervention, and thereby less chance of spreading the disease in regions with little access to modern healthcare.
Global health awareness is an essential factor for the market growth. Increasing global connection has made contagious diseases like the Zika virus appear to be everybody's threat these days. Rising interest in preventing and managing diseases, governments along with healthcare, have increased international focus on the aspect of public health. Thus, with the above measures, a strong demand is witnessed for accurate and reliable diagnostic tests to monitor the virus.
Public health initiatives focus more on surveillance systems that allow early detection since this is vital for controlling the spread of Zika, particularly in areas where mosquito-borne diseases are common. Large-scale screening programs and point-of-care testing in rural and underserved areas improve access and reduce diagnostic delays.
The severe complications associated with Zika have, however gained further momentum toward advanced testing. Thus, the need for testing methods such as molecular and serological tests has increased, involving PCR and ELISA, respectively. The international collaboration, as well as funding, toward the prevention of diseases has facilitated faster development and availability of testing solutions at reasonable prices.
In short, increased global health awareness of infectious diseases promotes this testing for Zika virus and has driven the impulse to advance diagnostic technology plus expand its access globally.
The global Zika virus testing market's compound annual growth rate (CAGR) for the first half of 2024 and 2025 is compared in the table below. This analysis provides important insights into the performance of the industry by highlighting significant shifts and trends in revenue generation.
The first half (H1) is the period from January to June, and the second half (H2) is July to December. In the first half (H1) of the decade from 2024 to 2034, the business is predicted to surge at a CAGR of 6.8%, followed by a slightly lower growth rate of 6.3% in the second half (H2) of the same decade.
| Particular | Value CAGR |
|---|---|
| H1 | 6.8% (2024 to 2034) |
| H2 | 6.3% (2024 to 2034) |
| H1 | 5.6% (2025 to 2035) |
| H2 | 5.1% (2025 to 2035) |
Moving into the subsequent period, from H1 2025 to H2 2035, the CAGR is projected to decrease slightly to 5.6% in the first half and remain relatively moderate at 5.1% in the second half. In the first half (H1) the industry witnessed a decrease of 110 BPS while in the second half (H2), the industry witnessed a decrease of 110 BPS.
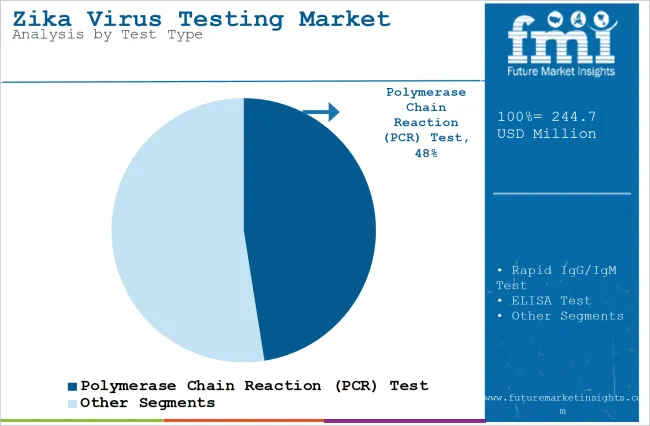
The section contains information about the leading segments in the industry. Based on test type, the polymerase chain reaction (PCR) test segment is expected to account for 47.5% of the global share in 2023.
| By Test Type | Polymerase Chain Reaction (PCR) Test |
|---|---|
| Value Share (2025) | 47.5% |
The polymerase chain reaction (PCR) test segment is projected to be a dominating segment in terms of revenue, accounting for almost 47.5% of the market share in 2025.
The PCR test segment is anticipated to dominate the market due to its unmatched accuracy, sensitivity, and reliability in detecting the virus. PCR permits amplification of viral RNA to enable detection at low viral loads, which is important for early-stage diagnosis. High sensitivity makes PCR the method of choice for confirmation of Zika virus infections, especially when other methods of diagnosis are not successful.
Moreover, PCR tests are fast, with results coming out in hours, which is very crucial during breakouts to contain and respond soon. It can detect the virus even if symptoms are mild or absent during the acute phase of infection, hence adding to its effectiveness in disease management.
As the healthcare sector across the world puts emphasis on rapid and precise diagnostics, proven performance and ability to deliver results, PCR emerges as the leader test in the market, particularly in clinical and laboratory settings.
| By End User | Hospitals & Clinics |
|---|---|
| Value Share (2025) | 49.7% |
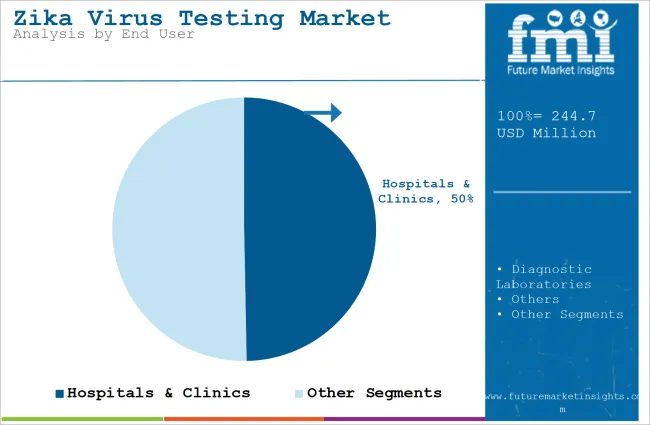
The hospitals & clinics segment will dominate the industry in terms of revenue, accounting for almost 49.7% of the market share in 2025.
The hospitals & clinics segment will dominate the industry because there is an increasing demand for accurate and fast diagnostics in health care settings. Hospitals and clinics are central sites for diagnosis and treatment of Zika virus infections, mainly for those whose symptoms and complications are serious and include pregnant women who risk being affected with congenital effects.
These settings require reliable testing methods to ensure quick and precise diagnoses, which is critical for patient management and outbreak control.
The presence of more advanced diagnostic equipment, including PCR and serological tests, in hospitals and clinics further increases the dominance of this segment. The established infrastructure, trained personnel, and easy access to healthcare resources in hospitals and clinics make it easier to implement testing protocols effectively.
As the infection control and preparedness measures of healthcare systems worldwide gain priority, the demand for Zika virus testing in hospitals and clinics increases, thus making it a prime driver in the market.
Rising prevalence of Zika Virus Infections is driving the market growth
Increasing prevalence of Zika virus infections is a major driver, majorly in the tropical and subtropical regions of the world. The outbreaks involving Zika virus in recent years had increased, mostly because the spreading rate of mosquito populations. The upsurge of reported cases indicates a greater and greater need to have an immediate and accurate diagnostics solution to gain control over such viruses.
Rapid diagnostic testing is important for early detection, especially in resource-poor settings where time-to-diagnosis may limit transmission. Increased number of cases highlighted the role of diagnostic testing for the management and control of outbreak and subsequent potential spread.
A surge in cases has driven technological innovation in terms of molecular tests, such as PCR, serological tests such as ELISA, and quick IgG and IgM detection tests.
With the increasing rate of infections, governments and health organizations are now investing in advanced diagnostic tools in the development and distribution process. The aim here is to enhance detection rates especially in high-risk areas, therefore reducing the burden of Zika virus infections. Thus, rising rates of Zika infections will drive the market growth.
Rising government initiatives and funding is driving the industry growth
Following the rising threat of Zika, governments and other international health authorities have made special efforts in fund allocations towards R&D for a diagnostic solution for this virus. Financial assistance also enhances the research of the tool with greater sensitivity, rapid response, and ease in its accuracy towards early-stage detection of this virus.
The governments often partner with public health agencies, non-governmental organizations, and private companies to ensure that the diagnostic technologies reach the markets most affected by Zika. This collaboration also fosters innovation, encouraging the development of advanced testing methods, such as PCR, ELISA, and rapid tests, which are essential for timely detection and outbreak management.
Government-funded funding programs also support the implementation of diagnostic tools by healthcare systems in developing countries into areas with fewer medical resources. These efforts will improve outbreak control and transmission control. As the governments continue to make resource allocations to detect Zika virus, these efforts are a continued impetus for the growth and adoption of testing technologies, thus expanding the market further.
Development of multiplex assays creates further growth opportunity
Multiplex assays represent a significant growth opportunity in the market. Combining Zika virus testing with diagnostics for other arboviruses, such as dengue and chikungunya, multiplex assays provide enhanced diagnostic efficiency. Healthcare providers can use these assays to detect multiple viruses from a single sample, thus saving time and resources while improving diagnostic accuracy in regions where these diseases are prevalent.
For example, it would be especially useful in the developing regions, where health care infrastructure is limited, where numerous outbreaks of various arboviruses occur often simultaneously. This integrated approach not only simplifies the diagnostic process but also increases the demand for multiplex testing solutions.
In addition, because public health agencies and hospitals want cost-effective, all-in-one diagnostic tools, multiplex assays can fill that gap by saving the number of individual tests required. The ever-increasing need for rapid, accurate, and cost-effective diagnostics further propels the market, making it a key opportunity for market growth in the Zika virus testing sector.
As such tests continue to evolve, they hold tremendous potential for enhanced disease detection and better management of outbreaks.
High cost of advanced diagnostic tests may restrict market growth
One of the most important deterrents to Zika virus testing is its expensive, high-end nature. Molecular tests, such as PCR, are often the most accurate methods for detecting Zika virus, whose operation cost could include equipment cost, cost of the reagent required for performing tests, and other cost. These costs can make it challenging for healthcare facilities in resource-limited areas to adopt these advanced technologies.
In low-income regions, where healthcare budgets are limited, the expense of molecular testing often leads to delays in diagnosis and limited access to accurate testing. This, in turn, hampers efforts to manage and contain Zika outbreaks effectively.
As a result, the market faces barriers in reaching underserved populations that would benefit most from early and accurate detection. The high cost also has a bearing on the comprehensive adoption of diagnostic tools, especially in areas where other health priorities compete for funding.
For instance, Zika Virus (ZIKV) TaqMan RT-PCR Detection Kits offered by Norgen Biotek Corp., cost around USD 749.0 for per kit.
Thus, such high of diagnostics test kits can hamper the market growth in near future.
The global Zika virus testing market recorded a CAGR of 4.7% during the historical period between 2020 and 2024. The growth of Zika virus testing industry was positive as it reached a value of USD 419.9 million in 2035 from USD 244.7 million in 2025.
Conventional methods of testing for viral infections range from viral culture, ELISA, and serological testing. These will typically take days to weeks to determine and rely more on the existence of antibodies or the antigens present. While reliable, the methods may not be so sensitive, particularly at the early stages of infection.
The newer PCR and NGS technologies can currently detect viruses, directly from samples of patients in a shorter duration and with high accuracy, providing diagnoses within hours instead of days and weeks.
Also, these cutting-edge technologies make it possible for infections to be detected at lesser viral loads than the traditional methods and are more sensitive when infections occur early in the cycle, thereby better diagnosing the cause of an illness at its nascent stages as well as outbreak management.
Major developments in molecular diagnostics such as real-time PCR and multiplex assays also boost the growth of the market. Real-time PCR amplifies the viral genetic material for faster, more accurate detection and provides results within hours. It is sensitive to the lowest possible viral load, allowing detection even at an early stage.
Multiplex assays further enhance diagnostic efficiency by enabling the simultaneous detection of multiple viruses, such as Zika, dengue, and chikungunya, from a single sample. This reduces the need for separate tests, saving time and costs while increasing the diagnostic capacity of healthcare systems.
These advancements improve diagnostic speed, accuracy, and cost-effectiveness, making them crucial for managing outbreaks and driving the demand for Zika virus testing solutions
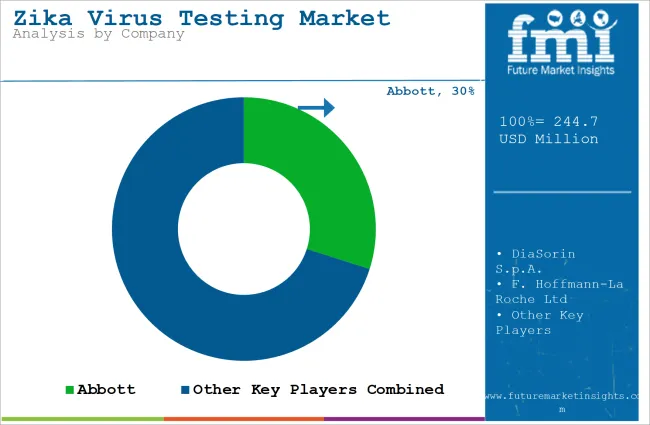
Tier 1 companies are the industry leaders with 44.3% of the global industry. These companies stand out for having a large product portfolio and a high production capacity. These industry leaders also stand out for having a wide geographic reach, a strong customer base, and substantial experience in manufacturing and having enough financial resources, which enables them to enhance their research and development efforts and expand into new industries.
The companies within tier 1 have a good reputation and high brand value. These companies frequently get involved in strategies such as acquisition and product launches. Prominent companies within tier 1 include Abbott, DiaSorin S.p.A., F. Hoffmann-La Roche Ltd and Quest Diagnostics
Tier 2 companies are relatively smaller as compared with tier 1 players. The tier 2 companies hold a market share of 28.7% worldwide. These firms may not have cutting-edge technology or a broad global reach, but they do ensure regulatory compliance and have good technology.
The players are more competitive when it comes to pricing and target niche markets. Key Companies under this category include Siemens Healthineers AG, Thermo Fisher Scientific and bioMérieux.
Compared to Tiers 1 and 2, Tier 3 companies offer Zika virus testing, but with smaller revenue spouts and less influence. These companies mostly operate in one or two countries and have limited customer base. The companies such as Altona Diagnostics, Clent Life Science, Norgen Biotek Corp and others falls under tier 3 category. They specialize in specific products and cater to niche markets, adding diversity to the industry.
The market analysis for Zika virus testing in various nations is covered in the section below. An analysis of important nations in North America, Latin America, Western Europe, Eastern Europe, East Asia, South Asia & Pacific, and Middle East & Africa of the world has been mentioned below.
It is projected that the United States will maintain its leading position in North America through 2035, holding a value share of 82.9%. By 2035, China is expected to experience a CAGR of 7.1% in the Asia-Pacific region.
| Countries | Value CAGR (2025 to 2035) |
|---|---|
| United States | 5.0% |
| Germany | 4.3% |
| UK | 4.6% |
| France | 5.0% |
| China | 7.1% |
| South Korea | 6.4% |
| India | 7.7% |
Germany’s Zika virus testing market is poised to exhibit a CAGR of 4.3% between 2025 and 2035. The Germany holds highest market share in European market.
In Germany, increased global health awareness has substantially pushed forward the adoption of Zika virus testing. As a superpower in public health, Germany has placed emphasis on preparedness and surveillance in managing infectious diseases.
Although cases of Zika outbreaks remain relatively small in Germany, the country's aggressive approach in responding to global health concerns spurred the adoption of diagnostic solutions to monitor the virus, especially among travelers returning from infected regions.
Advanced diagnostic tools such as PCR and serological tests, supported by the government and public funding, have been easily incorporated into routine practice in Germany. Public health agencies have also sought to improve the early detection capacity, especially in travelers, by running screening programs and surveillance systems.
The concern regarding Zika's possible complications, for instance, complications in pregnancy, has increased demand for rapid and accurate testing. As a result, Germany remains committed to the global health initiative and disease prevention. This drives the growth of Zika virus testing in the country.
United States is anticipated to show a CAGR of 5.0% between 2025 and 2035.
The increase in cases of Zika virus infection, mostly through outbreaks, has been highly observed in the tropical and subtropical regions, thereby creating the demand for a proper diagnostic method. Though it has been relatively rare in the United States, with an increase in travelers to areas affected by Zika, local transmission becomes a reality.
This has increased the demand for accurate and prompt diagnostic tools to identify the virus, especially in pregnant women because of the risks it poses to the fetus.
The USA healthcare system emphasizes early detection and outbreak control, and thus, public health authorities have implemented sophisticated testing techniques, including PCR and serological assays, to identify Zika infections as soon as possible.
Increasing awareness over Zika's impact on pregnancy is increasing demand for testing in clinical settings, which is boosting development in diagnostic technologies. The rising cases of Zika and a need for quick response mechanisms is further growing the US Zika virus testing market.
China is anticipated to show a CAGR of 7.1% between 2025 and 2035.
The main driver of the Chinese market is the demand for point-of-care testing. China is a very populous country with both urban and rural regions, where access to healthcare is difficult in remote and resource-limited regions. POC testing solutions are portable and easy to use, making them a practical way to address these challenges.
These tests would allow for rapid and accurate diagnoses with minimal need for sophisticated laboratory infrastructure, making them highly suitable for remote and underserved areas.
With the continuous increase in travelers visiting or returning from Zika-infected regions, there is even greater pressure placed on having efficient diagnostic methods with faster turnaround times. Chinese Government efforts to enhance health care infrastructure for the rural population have led to an increased interest in POC tests.
Since these tests may provide results within minutes, diagnostic delays can be minimized by having timely interventions and disease control. This emerging requirement for inexpensive, accessible, and affordable diagnostics fuels the China Zika virus testing market further.
Key market players in the Zika virus testing market adopt various growth strategies to strengthen their position. These include product innovation, such as developing advanced diagnostic tools like real-time PCR and multiplex assays to improve testing speed and accuracy.
Strategic partnerships and collaborations with government agencies and healthcare organizations help expand market reach and enhance product distribution. Additionally, companies focus on geographic expansion, targeting regions with high Zika virus incidence, particularly in tropical and subtropical areas.
Investment in research and development also enables the introduction of cost-effective, portable point-of-care testing solutions to cater to resource-limited settings.
Recent Industry Developments in Zika Virus Testing Market:
In terms of test type, the industry is divided into polymerase chain reaction (PCR) test, rapid IgG/IgM test and ELISA test.
In terms of end user, the industry is segregated into hospitals & clinics, diagnostic laboratories and others
Key countries of North America, Latin America, East Asia, South Asia & Pacific, Western Europe, Eastern Europe and Middle East and Africa (MEA) have been covered in the report.
The global Zika virus testing industry is projected to witness CAGR of 5.6% between 2025 and 2035.
The global Zika virus testing industry stood at USD 232.7 million in 2024.
The global Zika virus testing industry is anticipated to reach USD 419.9 million by 2035 end.
China is expected to show a CAGR of 7.1% in the assessment period.
The key players operating in the global Zika virus testing industry Abbott, DiaSorin S.p.A., F. Hoffmann-La Roche Ltd, Quest Diagnostics, Siemens Healthineers AG, Thermo Fisher Scientific, bioMérieux, Altona Diagnostics, Clent Life Science, Norgen Biotek Corp and Others.
Table 1: Global Market Value (US$ million) Forecast by Region, 2017 to 2033
Table 2: Global Market Volume (Units) Forecast by Region, 2017 to 2033
Table 3: Global Market Value (US$ million) Forecast by Test Type, 2017 to 2033
Table 4: Global Market Volume (Units) Forecast by Test Type, 2017 to 2033
Table 5: Global Market Value (US$ million) Forecast by End User, 2017 to 2033
Table 6: Global Market Volume (Units) Forecast by End User, 2017 to 2033
Table 7: North America & Europe Market Value (US$ million) Forecast by Country, 2017 to 2033
Table 8: North America & Europe Market Volume (Units) Forecast by Country, 2017 to 2033
Table 9: North America & Europe Market Value (US$ million) Forecast by Test Type, 2017 to 2033
Table 10: North America & Europe Market Volume (Units) Forecast by Test Type, 2017 to 2033
Table 11: North America & Europe Market Value (US$ million) Forecast by End User, 2017 to 2033
Table 12: North America & Europe Market Volume (Units) Forecast by End User, 2017 to 2033
Table 13: Latin America Market Value (US$ million) Forecast by Country, 2017 to 2033
Table 14: Latin America Market Volume (Units) Forecast by Country, 2017 to 2033
Table 15: Latin America Market Value (US$ million) Forecast by Test Type, 2017 to 2033
Table 16: Latin America Market Volume (Units) Forecast by Test Type, 2017 to 2033
Table 17: Latin America Market Value (US$ million) Forecast by End User, 2017 to 2033
Table 18: Latin America Market Volume (Units) Forecast by End User, 2017 to 2033
Table 19: Central America Market Value (US$ million) Forecast by Country, 2017 to 2033
Table 20: Central America Market Volume (Units) Forecast by Country, 2017 to 2033
Table 21: Central America Market Value (US$ million) Forecast by Test Type, 2017 to 2033
Table 22: Central America Market Volume (Units) Forecast by Test Type, 2017 to 2033
Table 23: Central America Market Value (US$ million) Forecast by End User, 2017 to 2033
Table 24: Central America Market Volume (Units) Forecast by End User, 2017 to 2033
Table 25: Caribbean Market Value (US$ million) Forecast by Country, 2017 to 2033
Table 26: Caribbean Market Volume (Units) Forecast by Country, 2017 to 2033
Table 27: Caribbean Market Value (US$ million) Forecast by Test Type, 2017 to 2033
Table 28: Caribbean Market Volume (Units) Forecast by Test Type, 2017 to 2033
Table 29: Caribbean Market Value (US$ million) Forecast by End User, 2017 to 2033
Table 30: Caribbean Market Volume (Units) Forecast by End User, 2017 to 2033
Table 31: South East Asia Market Value (US$ million) Forecast by Country, 2017 to 2033
Table 32: South East Asia Market Volume (Units) Forecast by Country, 2017 to 2033
Table 33: South East Asia Market Value (US$ million) Forecast by Test Type, 2017 to 2033
Table 34: South East Asia Market Volume (Units) Forecast by Test Type, 2017 to 2033
Table 35: South East Asia Market Value (US$ million) Forecast by End User, 2017 to 2033
Table 36: South East Asia Market Volume (Units) Forecast by End User, 2017 to 2033
Table 37: Rest of the World Market Value (US$ million) Forecast by Country, 2017 to 2033
Table 38: Rest of the World Market Volume (Units) Forecast by Country, 2017 to 2033
Table 39: Rest of the World Market Value (US$ million) Forecast by Test Type, 2017 to 2033
Table 40: Rest of the World Market Volume (Units) Forecast by Test Type, 2017 to 2033
Table 41: Rest of the World Market Value (US$ million) Forecast by End User, 2017 to 2033
Table 42: Rest of the World Market Volume (Units) Forecast by End User, 2017 to 2033
Figure 1: Global Market Value (US$ million) by Test Type, 2023 to 2033
Figure 2: Global Market Value (US$ million) by End User, 2023 to 2033
Figure 3: Global Market Value (US$ million) by Region, 2023 to 2033
Figure 4: Global Market Value (US$ million) Analysis by Region, 2017 to 2033
Figure 5: Global Market Volume (Units) Analysis by Region, 2017 to 2033
Figure 6: Global Market Value Share (%) and BPS Analysis by Region, 2023 to 2033
Figure 7: Global Market Y-o-Y Growth (%) Projections by Region, 2023 to 2033
Figure 8: Global Market Value (US$ million) Analysis by Test Type, 2017 to 2033
Figure 9: Global Market Volume (Units) Analysis by Test Type, 2017 to 2033
Figure 10: Global Market Value Share (%) and BPS Analysis by Test Type, 2023 to 2033
Figure 11: Global Market Y-o-Y Growth (%) Projections by Test Type, 2023 to 2033
Figure 12: Global Market Value (US$ million) Analysis by End User, 2017 to 2033
Figure 13: Global Market Volume (Units) Analysis by End User, 2017 to 2033
Figure 14: Global Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 15: Global Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 16: Global Market Attractiveness by Test Type, 2023 to 2033
Figure 17: Global Market Attractiveness by End User, 2023 to 2033
Figure 18: Global Market Attractiveness by Region, 2023 to 2033
Figure 19: North America & Europe Market Value (US$ million) by Test Type, 2023 to 2033
Figure 20: North America & Europe Market Value (US$ million) by End User, 2023 to 2033
Figure 21: North America & Europe Market Value (US$ million) by Country, 2023 to 2033
Figure 22: North America & Europe Market Value (US$ million) Analysis by Country, 2017 to 2033
Figure 23: North America & Europe Market Volume (Units) Analysis by Country, 2017 to 2033
Figure 24: North America & Europe Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 25: North America & Europe Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 26: North America & Europe Market Value (US$ million) Analysis by Test Type, 2017 to 2033
Figure 27: North America & Europe Market Volume (Units) Analysis by Test Type, 2017 to 2033
Figure 28: North America & Europe Market Value Share (%) and BPS Analysis by Test Type, 2023 to 2033
Figure 29: North America & Europe Market Y-o-Y Growth (%) Projections by Test Type, 2023 to 2033
Figure 30: North America & Europe Market Value (US$ million) Analysis by End User, 2017 to 2033
Figure 31: North America & Europe Market Volume (Units) Analysis by End User, 2017 to 2033
Figure 32: North America & Europe Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 33: North America & Europe Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 34: North America & Europe Market Attractiveness by Test Type, 2023 to 2033
Figure 35: North America & Europe Market Attractiveness by End User, 2023 to 2033
Figure 36: North America & Europe Market Attractiveness by Country, 2023 to 2033
Figure 37: Latin America Market Value (US$ million) by Test Type, 2023 to 2033
Figure 38: Latin America Market Value (US$ million) by End User, 2023 to 2033
Figure 39: Latin America Market Value (US$ million) by Country, 2023 to 2033
Figure 40: Latin America Market Value (US$ million) Analysis by Country, 2017 to 2033
Figure 41: Latin America Market Volume (Units) Analysis by Country, 2017 to 2033
Figure 42: Latin America Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 43: Latin America Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 44: Latin America Market Value (US$ million) Analysis by Test Type, 2017 to 2033
Figure 45: Latin America Market Volume (Units) Analysis by Test Type, 2017 to 2033
Figure 46: Latin America Market Value Share (%) and BPS Analysis by Test Type, 2023 to 2033
Figure 47: Latin America Market Y-o-Y Growth (%) Projections by Test Type, 2023 to 2033
Figure 48: Latin America Market Value (US$ million) Analysis by End User, 2017 to 2033
Figure 49: Latin America Market Volume (Units) Analysis by End User, 2017 to 2033
Figure 50: Latin America Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 51: Latin America Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 52: Latin America Market Attractiveness by Test Type, 2023 to 2033
Figure 53: Latin America Market Attractiveness by End User, 2023 to 2033
Figure 54: Latin America Market Attractiveness by Country, 2023 to 2033
Figure 55: Central America Market Value (US$ million) by Test Type, 2023 to 2033
Figure 56: Central America Market Value (US$ million) by End User, 2023 to 2033
Figure 57: Central America Market Value (US$ million) by Country, 2023 to 2033
Figure 58: Central America Market Value (US$ million) Analysis by Country, 2017 to 2033
Figure 59: Central America Market Volume (Units) Analysis by Country, 2017 to 2033
Figure 60: Central America Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 61: Central America Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 62: Central America Market Value (US$ million) Analysis by Test Type, 2017 to 2033
Figure 63: Central America Market Volume (Units) Analysis by Test Type, 2017 to 2033
Figure 64: Central America Market Value Share (%) and BPS Analysis by Test Type, 2023 to 2033
Figure 65: Central America Market Y-o-Y Growth (%) Projections by Test Type, 2023 to 2033
Figure 66: Central America Market Value (US$ million) Analysis by End User, 2017 to 2033
Figure 67: Central America Market Volume (Units) Analysis by End User, 2017 to 2033
Figure 68: Central America Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 69: Central America Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 70: Central America Market Attractiveness by Test Type, 2023 to 2033
Figure 71: Central America Market Attractiveness by End User, 2023 to 2033
Figure 72: Central America Market Attractiveness by Country, 2023 to 2033
Figure 73: Caribbean Market Value (US$ million) by Test Type, 2023 to 2033
Figure 74: Caribbean Market Value (US$ million) by End User, 2023 to 2033
Figure 75: Caribbean Market Value (US$ million) by Country, 2023 to 2033
Figure 76: Caribbean Market Value (US$ million) Analysis by Country, 2017 to 2033
Figure 77: Caribbean Market Volume (Units) Analysis by Country, 2017 to 2033
Figure 78: Caribbean Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 79: Caribbean Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 80: Caribbean Market Value (US$ million) Analysis by Test Type, 2017 to 2033
Figure 81: Caribbean Market Volume (Units) Analysis by Test Type, 2017 to 2033
Figure 82: Caribbean Market Value Share (%) and BPS Analysis by Test Type, 2023 to 2033
Figure 83: Caribbean Market Y-o-Y Growth (%) Projections by Test Type, 2023 to 2033
Figure 84: Caribbean Market Value (US$ million) Analysis by End User, 2017 to 2033
Figure 85: Caribbean Market Volume (Units) Analysis by End User, 2017 to 2033
Figure 86: Caribbean Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 87: Caribbean Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 88: Caribbean Market Attractiveness by Test Type, 2023 to 2033
Figure 89: Caribbean Market Attractiveness by End User, 2023 to 2033
Figure 90: Caribbean Market Attractiveness by Country, 2023 to 2033
Figure 91: South East Asia Market Value (US$ million) by Test Type, 2023 to 2033
Figure 92: South East Asia Market Value (US$ million) by End User, 2023 to 2033
Figure 93: South East Asia Market Value (US$ million) by Country, 2023 to 2033
Figure 94: South East Asia Market Value (US$ million) Analysis by Country, 2017 to 2033
Figure 95: South East Asia Market Volume (Units) Analysis by Country, 2017 to 2033
Figure 96: South East Asia Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 97: South East Asia Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 98: South East Asia Market Value (US$ million) Analysis by Test Type, 2017 to 2033
Figure 99: South East Asia Market Volume (Units) Analysis by Test Type, 2017 to 2033
Figure 100: South East Asia Market Value Share (%) and BPS Analysis by Test Type, 2023 to 2033
Figure 101: South East Asia Market Y-o-Y Growth (%) Projections by Test Type, 2023 to 2033
Figure 102: South East Asia Market Value (US$ million) Analysis by End User, 2017 to 2033
Figure 103: South East Asia Market Volume (Units) Analysis by End User, 2017 to 2033
Figure 104: South East Asia Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 105: South East Asia Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 106: South East Asia Market Attractiveness by Test Type, 2023 to 2033
Figure 107: South East Asia Market Attractiveness by End User, 2023 to 2033
Figure 108: South East Asia Market Attractiveness by Country, 2023 to 2033
Figure 109: Rest of the World Market Value (US$ million) by Test Type, 2023 to 2033
Figure 110: Rest of the World Market Value (US$ million) by End User, 2023 to 2033
Figure 111: Rest of the World Market Value (US$ million) by Country, 2023 to 2033
Figure 112: Rest of the World Market Value (US$ million) Analysis by Country, 2017 to 2033
Figure 113: Rest of the World Market Volume (Units) Analysis by Country, 2017 to 2033
Figure 114: Rest of the World Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 115: Rest of the World Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 116: Rest of the World Market Value (US$ million) Analysis by Test Type, 2017 to 2033
Figure 117: Rest of the World Market Volume (Units) Analysis by Test Type, 2017 to 2033
Figure 118: Rest of the World Market Value Share (%) and BPS Analysis by Test Type, 2023 to 2033
Figure 119: Rest of the World Market Y-o-Y Growth (%) Projections by Test Type, 2023 to 2033
Figure 120: Rest of the World Market Value (US$ million) Analysis by End User, 2017 to 2033
Figure 121: Rest of the World Market Volume (Units) Analysis by End User, 2017 to 2033
Figure 122: Rest of the World Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 123: Rest of the World Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 124: Rest of the World Market Attractiveness by Test Type, 2023 to 2033
Figure 125: Rest of the World Market Attractiveness by End User, 2023 to 2033
Figure 126: Rest of the World Market Attractiveness by Country, 2023 to 2033






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Norovirus Infection Treatment Market
Retrovirus Testing Market Size and Share Forecast Outlook 2025 to 2035
Coronavirus Immunoassay Market Size and Share Forecast Outlook 2025 to 2035
Nipah Virus (NiV) Infection Testing Market Insights by Test Type, End User, and Region through 2035
Marburg Virus Disease Therapeutics Market - Growth & Vaccine Advances 2025 to 2035
Coxsackievirus Infections Treatment Market – Growth & Drug Innovations 2025 to 2035
Cytomegalovirus Treatment Market Size and Share Forecast Outlook 2025 to 2035
Oncolytic Virus Cancer Therapy Market Growth – Innovations & Trends 2025 to 2035
West Nile Virus Testing Market
Hepatitis C Virus (HCV) Testing Market Size and Share Forecast Outlook 2025 to 2035
Oncolytic Adenovirus Market Size and Share Forecast Outlook 2025 to 2035
Avian Metapneumovirus Treatment Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Biotherapeutics Virus Removal Filters Market Trends – Growth & Forecast 2025 to 2035
Human Papilloma Virus Testing Market Size and Share Forecast Outlook 2025 to 2035
Chronic Hepatitis B Virus Testing Market Size and Share Forecast Outlook 2025 to 2035
Human Immunodeficiency Virus Type 1 (HIV 1) Market Size and Share Forecast Outlook 2025 to 2035
Human RSV Treatment Market Insights - Innovations & Forecast 2025 to 2035
Paediatric Respiratory Syncytial Virus Infection Market Growth - Trends & Forecast 2025 to 2035
Testing, Inspection & Certification Market Growth – Trends & Forecast 2025 to 2035
5G Testing Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA