Over the years, the burden of neurological diseases is increasing in emerging countries. According to estimates by the WHO, 4.5-11% of illnesses in both low- and high-income economies is triggered due to neurological disorders. The situation is more critical as compared to the patients suffering from respiratory ailments, gastrointestinal disorders, or cancers.
It is expected that the burden is expected to increase further over the forecast period. Neurological failure can also lead to permanent paralysis or disability, which becomes burden not only for the patients but also to their families. To overcome such complex situations, Vagus Nerve Stimulator therapy for last decades has been in use to overcome the improving neurological tremors, and dyskinesia, among others.
This system creates muscle contraction and is able to restore functionality of motor nerve. Besides, vagus nerve stimulators can also be used in patients for bladder control, restoration of hand grasp, and movement. Vagus nerve stimulator can be used for normalizing other neurological disorders such as migraines, Crohn's disease, Asthma, depression, headaches and cardiovascular diseases.
Don't pay for what you don't need
Customize your report by selecting specific countries or regions and save 30%!
Over the last two decades, treatment and gene delivery using vectors as carrier have been in peak of research to treat various neurological disorders. Also, vector-based gene modification have undergone remarkable changes against rising demand for transgene delivery to the host. For instance, vector development to treat patients with nervous system disorders using gene-induced vectors have undergone massive advancements.
Additionally, other vectors, e.g., lentiviral vector, has also been used widely in CNS gene therapy, as the vector has a larger transgene capacity. Besides, Herpes simplex virus and adenoviral vectors have also been utilized to treat CNS disorders. With advancements in next generation technology, it could possibly replace the shortcomings of implantable vagus nerve stimulators.
Over the past decades, it is been observed that Implantable VNS is associated with surgically related infections and abnormal complexities such as dysrhythmia, cough, paresthesia, pain and voice alteration, which gradually decreases the prevalence over time.
To overcome such side effects of implantable vagus nerve stimulator, companies such as Cerbomed and Electrocore in this decade have made commendable progress in their product research to introduce noninvasive vagus nerve stimulators to the world.
The devices were initially introduced in European region. Gradually, with recent FDA approvals and clearance for noninvasive vagus nerve stimulator product marketing and licensing, the devices have made strides and being accepted globally in various neurological treatment.
It is expected that the external vagus nerve stimulator is expected to provide an advanced hygienic and noninfectious profile as it does not requires implantation. Additionally, the external VNS devices are more effective in terms of treatment and are also affordable for patients.
In a research study, it is reported that 105 patients receiving vagus nerve stimulator system treatment from 1999 to 2008, twenty (19%) patients had encountered device-related technical failures and complications. Amongst device failures, first the device was removed in 8 cases.
Others also encountered some of the device-associated complications such as electrode malfunction, onset of wound infections & cardiac arrhythmia and posttraumatic dysfunction of the stimulator.
Vagus nerve stimulator therapy is also associated with wide array of possible complications. Other simultaneous technical failures include malfunction of dislocation, and generator. The major complication occurs in children, and the primary reason being electrode fracture, which might be induced by growth during the adolescence stage.
Once the neurologist prescribes vagus nerve stimulation therapy as one option for treatment of any neurological disorder, there comes this question as how much this treatment costs. The cost of vagus nerve stimulator therapy currently is high, including both the implant and surgical procedure. Costs of surgery and post-surgical care may vary in different parts of the world and may further increase over time.
Many insurance companies, such as Medicare and most Medicaid carriers, in the West cover almost all the costs of the vagus nerve stimulator therapy. However, people qualifying for vagus nerve stimulator therapy but without insurance cover or external funds need to pay the whole amount for the implant, which becomes a long-term burden for the patient.
This section of the report features profiles of key players operating in the vagus nerve stimulator market based on their market shares, differential strategies, vagus nerve stimulator product offerings, marketing approach and company dashboard. Examples of some of the key players featured in this report include ElectroCore LivaNova LLC, Cerbomed GmbH, ReShape Lifesciences, Inc., and NERVANA LLC, among others.
The key manufacturers of vagus nerve stimulator are focusing on marketing strategies to increase both their product portfolio as well as geographical presence. The company’s manufacturing vagus nerve stimulator are focused on pricing strategies in order to lead in the market for vagus nerve stimulator.
Some other key market strategies followed by vagus nerve stimulator manufacturers include lucrative discounts, strong distribution network for consumables and equipment, partnering with local as well as global retailers, and long-term partnerships with pathology laboratories, hospitals and contract research organizations.
Get the data you need at a Fraction of the cost
Personalize your report by choosing insights you need
and save 40%!
Implantable vagus nerve stimulator is a neuromodulation device, which is surgically implanted inside the skin of the chest, and a wire is inserted under the skin connecting the device to the left vagus nerve. Transcutaneous vagus nerve stimulator (tVNS) or noninvasive vagus nerve stimulation devices do not require any surgical implantation. The devices are handheld and used to treat epilepsy, pain-management and pain.
FMI conducted a research study on vagus nerve stimulator marketfor the forecast period 2018 to 2028. The vagus nerve stimulator marketreport offers a comprehensive evaluation of the business opportunities prevailing in the vagus nerve stimulator marketalong with insights on the vagus nerve stimulator, trend, device prices and awareness level for vagus nerve stimulator marketcompetition.
The report elaborates the macroeconomic factors influencing the dynamics of vagus nerve stimulator marketand its futuristic potential.
Some of the additional questions addressed in this report on vagus nerve stimulator market-
The vagus nerve stimulator markethas been estimated based on supply-demand approach. The market was first calculated based on vagus nerve stimulator volume conducted in different region/countries. The test volume was estimated based on instrument installation in top 20 countries globally. Other qualitative factors analyzed during test volume estimation include awareness level neurological disorders.
This information was further validated with rigorous primary research (including interviews, surveys, in-person interactions, and viewpoints of seasoned analysts) and secondary research (including verified paid sources, authentic trade journals, and resourceful databases).
The research study on vagus nerve stimulator marketalso includes top trends and macro as well as micro-economic factors shaping the vagus nerve stimulator market. With this approach, the report on vagus nerve stimulator marketanticipates the industry attractiveness of every major segment in the vagus nerve stimulator market over the forecast period.
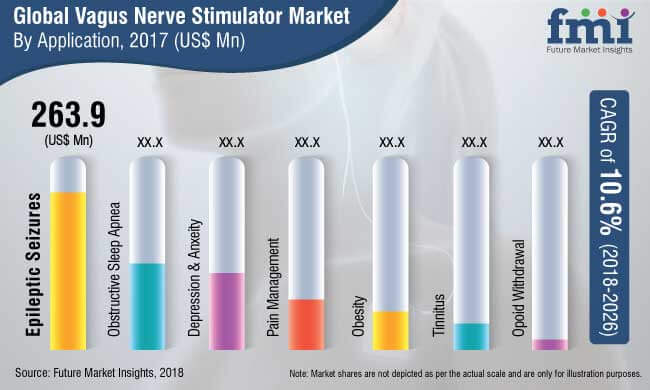
The report offers a comprehensive taxonomy of vagus nerve stimulator marketbased on product type, end user, application type and region. Based on product type, the vagus nerve stimulator market is segmented into implantable and noninvasive (tVNS) based. The vagus nerve stimulator finds application in pain management, epileptic seizures, obesity management, depression and anxiety.
These vagus nerve stimulators are used by hospitals, ambulatory surgical centers, specialty clinics and home care settings. The vagusnerve stimulator markethas been analysed across regions of North America, Latin America, Europe, Asia Pacific Excluding Japan, Japan and MEA.
The vagus nerve stimulator market is likely to register a CAGR of 9.89% during the forecast period.
The major complication occurs in children during the adolescent stage due to electrode fracture induced by growth.
By 2032, the market is likely to grow to a revenue of USD 1,647.43 Million.
North America holds the leading market share of vagus nerve stimulators.
1. Executive Summary
2. Market Introduction
3. Market Dynamics
4. Key Inclusions
5. Global Market Analysis 2013 to 2017 and Forecast 2018 to 2028
5.1. North America
5.2. Latin America
5.3. Europe
5.4. Asia Pacific excluding Japan
5.5. Japan
5.6. Middle East and Africa
6. North America Market Analysis 2013 to 2017 and Forecast 2018 to 2028
7. Europe Market Analysis 2013 to 2017 and Forecast 2018 to 2028
8. Latin America Market Analysis 2013 to 2017 and Forecast 2018 to 2028
9. Asia Pacific excluding Japan (APEJC) Market Analysis 2013 to 2017 and Forecast 2018 to 2028
10. Japan Market Analysis 2013 to 2017 and Forecast 2018 to 2028
11. MEA Market Analysis 2013 to 2017 and Forecast 2018 to 2028
12. Forecast Assumptions
13. Competition Analysis
13.1. ElectroCore
13.2. LivaNova LLC
13.3. Cerbomed GmbH
13.4. NERVANA LLC
13.5. ReShape Lifesciences, Inc
13.6. Beijing PINS Medical Co., Ltd.
13.7. Parasym Health
13.8. Innovative Health Solutions INC
14. Research Methodology
Explore Healthcare Insights
View Reports