The United States preclinical medical device testing services market is predicted to be 1,696.0 USD million in the year 2025. Going ahead, market is predicted to grow at an approximate growth of 5.3% consistent CAGR up to the estimated year of 2035 when the market shall be around USD 2,842.1 million.
| Attributes | Values |
|---|---|
| Estimated Industry Size 2025 | USD 1,696.0 million |
| Projected Value 2035 | USD 2,842.1 million |
| Value-based CAGR from 2025 to 2035 | 5.3% |
The United States preclinical medical device testing services market is expected to witness significant growth. Factors such as advancements in various medical technologies, increasing pressure of regulatory control, and high consideration towards patients' safety attribute to the growth of the market.
The market is further driven by growing demand for faster development of medical device products and the requirement of regulatory compliance by authorities. Further, innovations in simulation, imaging, and different technologies have improved efficiency and precision of the preclinical test, allowing the reduction of overall time-to-market cost. Furthermore, growing emphasis of manufacturers on adopting sustainable practices with a major focus on introducing patient-centric solution significantly attribute to the growth of the market.
Explore FMI!
Book a free demo
The table below offers a detailed comparative assessment of the changes in the compound annual growth rate (CAGR) over six months for the base year (2024) and the current year (2025) specifically for the United States preclinical medical device testing services market. This semi-annual analysis highlights demonstrates a changes in market dynamics. The H1, which is the first half of the year shows the January to June, while the second half, H2, spans July to December.
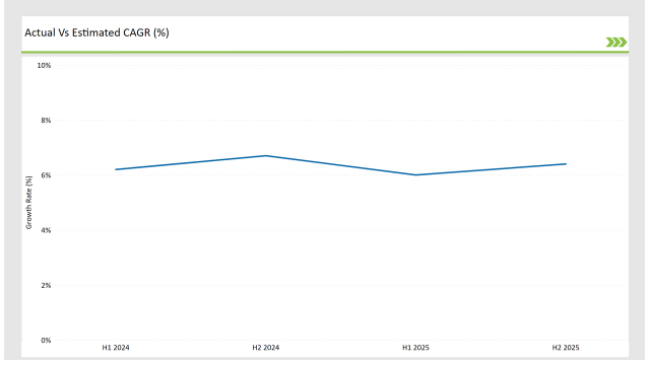
H1 signifies period from January to June, H2 Signifies period from July to December
Preclinical medical device testing services is projected to grow at a CAGR of 6.2% during H1 2024 and further surge to an increment of 6.7% in the latter half of 2024. In 2024, the rate is projected to slightly lower down to 6.0% in H1 and increase up to 6.4% in H2.
The market also witnessed decline of 20 basis points from the first half of 2024 to the first half of 2025 and an increase of 25 basis points in the second half of 2025 over the second half of 2024. The foregoing figures describe the dynamics or changing nature in the Germany preclinical medical device testing services market impacted by factors, such as altered regulatory changes or innovations in several services provided by the service providers.
| Date | Development/M&A Activity & Details |
|---|---|
| 2025 | Growing Number of Service Providers: America® Holdings and NAMSA is responsible for the increase in number of Preclinical Medical Device Testing services in the USA which will anticipate the growth of the market |
| 2024 | Introduction of Improved Biocompatibility Testing: Intertek Group Plc is continuously emphasizing on introducing tests that are biocompatible and focusing on expanding their service portfolio. |
| 2024 | Advancements in In-vitro Testing: WUXI APPTEC company is responsible for the significant progress made by in-vitro testing methods anticipate the growth of the market. |
Growing Demand of Medical Devices anticipates the Growth of Market in the USA
Rising prevalence of chronic diseases such as diabetes, cardiovascular conditions, and neurological disorders contribute to the growing demand of medical devices which in turn significantly drives the growth of the market in the USA Furthermore, growing complexity and the drive toward speedier time-to-market fuel demands for resourceful and accurate test services.
In newer technologies, the integration of AI, 3D modeling, and in-vitro testing methods has further enhanced one's capability to test and made it more cost-effective and rapid. The need to establish safe and dependable medical devices has, therefore, made preclinical test services an important requirement in driving the overall market.
Preferential Shift towards Minimal Invasive Procedures Surges Market Growth in USA
The transition toward minimal invasiveness of procedures and wearable health gadgets is a major reason for the growth in the preclinical medical device testing services market. Devices have grown smaller, more complex, and integrated with advanced technologies that require specialized preclinical testing to ensure their safety, effectiveness, and biocompatibility. These are long-term-performing devices when used by patients, and they must be thoroughly vetted to ensure that they are effective without causing harm.
Preclinical testing services form an integral constituent of the process in determining device functionality, durability, and associated risks before they can be used in clinical trials. The demand for these devices increases, and so does the requirement for accuracy and comprehensive testing services to further expand the market, ensuring that new and innovative devices meet regulatory requirements and deliver positive patient outcomes.
% share of Individual categories by Service Type and End User in 2025
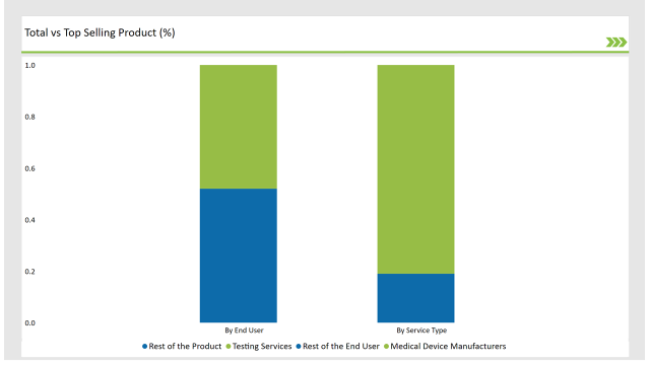
The Critical Factor of Ensuring the Safety and Efficiency in Medical Device Aid Testing Services to dominate the Market
Before clinical trials or entry into the market, devices need to be tested to ascertain their performance, and risks that is associated with them. Devices are put through rigorous preclinical testing by regulatory bodies like the FDA and EMA to ensure safety standards, thus increasing the demand for such services.
Because the nature of medical devices becomes more complex with every passing day, in-vitro testing, in vivo testing, and mechanical stress testing are specialized forms of testing to replicate field conditions. These testing services allow a manufacturer to reduce the risk of costly product failure, achieve regulatory deadlines, and provide readiness for patient use, making it a key part of the product development process.
Comprehensive Testing Required by Medical Device aid them to hold Dominant Position
Medical device manufacturers are the largest portion of the preclinical medical device testing services market, as they generally bear responsibility for the development, design, and bringing to market of medical devices. Such a manufacturer has to undergo comprehensive testing to ensure their devices meet the standards for safety, efficacy, and regulatory use before they can be taken into clinical trials or sold to the public.
Preclinical testing identifies risks, performance issues, and compliance gaps early in the course of the development cycle and saves manufacturers time and money.
Also, most of the medical device manufacturing companies have solid connections with special testing service providers for wearable, implantable, or diagnostic applications. In partnership with service providers, this can accelerate development for a manufacturer keen to meet regulatory deadlines and advance safe, effective products to market-a path to leading positions in the preclinical testing market.
The United States preclinical medical device testing services market is moderately fragmented, with a mix of multinational corporations and regional players contributing to a dynamic competitive environment. Companies like Laboratory Corporation of America Holdings, Charles River Laboratories, WUXI APPTEC and Sotera Health dominate the market by leveraging advanced technologies for streamlining their production process.
The competitive landscape of the United States preclinical medical device testing services market features a blend of major multinational corporations and innovative regional companies.
2025 Market share of USA Preclinical Medical Device Testing Services
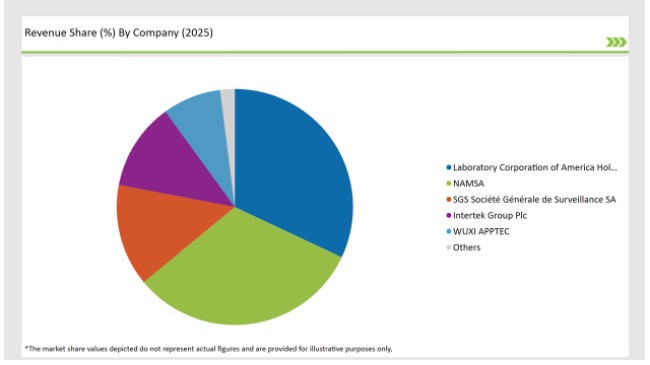
Note: above chart is indicative in nature
By 2025, the United States preclinical medical device testing services market is expected to grow at a CAGR of 5.3%.
By 2035, the sales value of the United States preclinical medical device testing services industry is expected to reach USD 2,842.1 million.
Key factors that are attributing to the growth of the United States preclinical medical device testing services market include higher regulatory scrutiny, and emphasis of manufacturer on introducing their product to the market in reduced time.
Prominent players in the United States preclinical medical device testing services manufacturing include Laboratory Corporation of America® Holdings, NAMSA, SGS Société Générale de Surveillance SA., Intertek Group Plc, WUXI APPTEC, TÜV SÜD, Sotera Health, Eurofins Scientific, iuvo BioScience, llc, RQM+, Pace Analytical Services LLC, Pharmaron, Bioneeds India Pvt. Ltd., Porsolt, Gradient LLC and Goupe Icare.
The industry includes Testing Services (Biocompatibility Testing, Microbiological & Sterility Testing, Analytical chemistry {Material Characterization, Extractables and leachables, Storage and stability testing and Polymer Investigation}, Toxicology Testing { Cytotoxicity, Genotoxicity and Other Toxicology Testing}, Functional Testing, Electromagnetic Compatibility (EMC) Testing, Implantation Studies, Biological Safety Evaluation, Package Validation, Reusability Testing, Pyrogen Testing and Others, and Consulting Services (Device Designing/Engineering and Regulatory affairs Consulting).
In terms of device category, the industry is divided into Orthopedics, Cardiovascular, Respiratory, Diabetes, Dental, Neurology, Oncology, Ocular, Bariatrics, Wound Healing, General Health (Wearables), In Vitro Diagnostics, General Surgery, Drug Device Combination and Other Device Category.
The industry is divided into Class I, Class II and Class III.
The industry is classified by end user as medical device manufacturers, pharmaceutical and biotech companies, device design and engineering firms and academic and research institutions
Glaucoma Treatment Market Overview – Trends & Forecast 2025 to 2035
Gastrointestinal Stent Market Growth – Trends & Forecast 2025 to 2035
Gel Implants Market Analysis - Trends, Share & Forecast 2025 to 2035
Gaucher and Pompe Diseases Enzyme Replacement Therapy (ERT) Market - Growth & Forecast 2025 to 2035
3D Printing Dental Devices Market Growth - Trends & Forecast 2025 to 2035
3D Printed Hip and Knee Implants Market Growth - Trends & Forecast 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.