Thyroid Function Test (TFT) Market is expected to develop exponentially from 2025 through 2035 based on thyroid-related disorders' growth, raised health and healthcare-related awareness, and developments in diagnosis and testing. Thyroid diagnostic tests aid the physician in precise diagnosis in hypothyroidism as well as in hyperthyroidism conditions and issues therewith and assist effective medical treatment for hypothyroidism and the above cases.
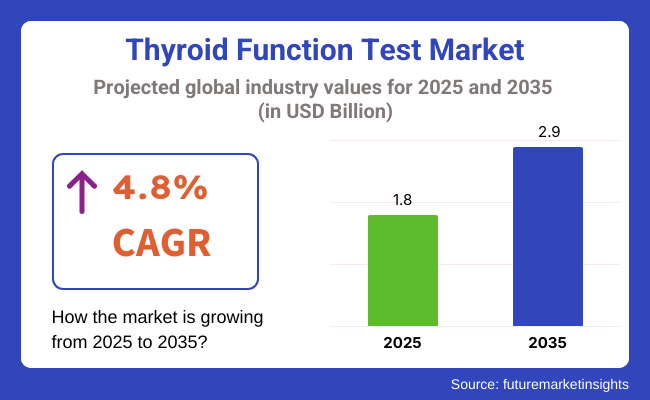
The market in 2025 is USD 1.8 billion and will be USD 2.9 billion in 2035 at a CAGR of 4.8%. Demand for the market is expected to be driven primarily by the increasing prevalence of autoimmune disorders such as Hashimoto's thyroiditis and Graves' disease and lifestyle factors including stress and iodine deficiency.
Rapid adoption and availability of point-of-care testing(POCT), home testing and AI-enabled diagnostic platforms are reshaping the market dynamics Test speed, performance and availability are fuelled by the confluence of state-of-the-art immunoassays technologies, biomarker discovery and automation in labs.
Government measures to enhance the awareness of thyroid disorders, reimbursement for diagnostic processes from insurance providers, and expanding R&D expenses on precision medicine are expected to fuel the growth of the market over the course of the forecasting period.
Explore FMI!
Book a free demo
North America and Europe will jointly contribute the highest market share in the market for thyroid function testing due to high healthcare expenditure, uptick in awareness for thyroid disorders, and developed well diagnostic infrastructure.
Thyroid disease diagnosis and treatment began in the USA and Canada, along with the rise in obesity, demographic aging, and increased incidence of autoimmune diseases with consequent thyroid dysfunction. The American Thyroid Association (ATA) as well as other health organizations help to generate awareness, promote screening programs, and fund research related to thyroid health.
Furthermore, the clinical adoption regarding thyroid function tests is further fuelled by advances in high-sensitivity thyroid-stimulating hormone (TSH) assays and next generation sequencing (NGS) for thyroid cancers. North American market growth in the future is expected to be driven by the expansion of telemedicine services, the availability of thyroid testing kits in the home setting, and the approval of new in-vitro diagnostics (IVDs) by the FDA.
Germany, the United Kingdom, and France maintain a principal share of the thyroid function test market owing to their advanced diagnostic innovations, comprehensive healthcare capabilities, and progressive regulatory frameworks. The growing incidence of thyroid disorders, especially among women and older adults, is increasing the need for routine thyroid screening and hormone level testing.
Growth in the adoption of automated diagnostic equipment, digital pathology solutions, and AI-driven laboratory workflows is improving the accuracy and efficiency of thyroid function tests in Europe. In addition, government initiatives promoting early detection of diseases as well reimbursement policies for diagnostic testing are supporting the market growth.
Europe is well stocked with research funding in endocrinology and biomarker-driven disease profiling to drive precision medicine for the management of thyroid dysfunction. Emphasis on personalized treatment strategies and integration of genetic testing for thyroid disorders in the region is anticipated to create new opportunities for the market players.
Asia-Pacific region is anticipated to grow the most in the thyroid function tests market during 2023, increasing healthcare expenditure, and growing awareness associated with the thyroid health to drive this market across China, India, Japan and South Korea.
Increased urbanization, shifting dietary patterns, and environmental conditions that lead to iodine deficiency have contributed to growing prevalence of thyroid disorders in the region. Market adoption is being driven by expanding healthcare infrastructure, government-backed screening programs, and the private sector's investment in diagnostic labs.
Rapid growth of diagnostic laboratory services, expansion of medical tourism, and increased adoption of advanced immunoassays for the analysis of thyroid functions are contributing to the growth of the market in emerging economies particularly China, and India. Urbanized areas are also expanding the penetration of home diagnostic tests due to the emergence of telemedicine and at-home diagnostic testing solutions.
With an emphasis on research and development in endocrinology and thyroid oncology, Japan and South Korea are leading the way in innovative solutions for detection of early thyroid cancer and hormone imbalance assessment.
Challenges
Opportunities
Thyroid function tests 2020 to 2024 were however increasing at high speed given the incidence rates of our section in the course of over hundreds years in addition to the importance of endocrine health, and advances in diagnostic sciences. Over the past few decades, the need for early and accurate diagnosis has been Raman as the prevalence of disorders like hypothyroidism, hyperthyroidism, Hashimoto's thyroiditis, and thyroid cancer continues to rise.
Screening and monitoring thyroid function tests (TFTs) were performed by physicians for levels of thyroid-stimulating hormone (TSH), free thyroxine (FT4), free triiodothyronine (FT3), and thyroid peroxidase antibodies (TPOAbs), which were essential in diagnosing and monitoring treatment of both thyroid disease and autoimmune thyroid disease.
Increasing incidence of autoimmune diseases, iodine deficiency, and endocrine imbalance rendered highly sensitive and specific thyroid testing kits unavoidable. Further orders were made for low-trauma, cost-effective, and rapid thyroid function test solutions by laboratory diagnostic services, hospitals, and home health agencies in order to further promote levels of screening and outcome in patients.
Advancements in technology (i.e., chemiluminescent immunoassays [CLIA], electrochemiluminescence immunoassays [ECLIA], and high-throughput ELISA-based thyroid assays) enhanced detection sensitivity and specificity. AI-based thyroid wellness monitoring platforms were designed by integrating big data analytics and machine learning algorithms to monitor the real-time biomarker fluctuations. Now, wearable biosensors capable of monitoring thyroid hormone levels were developed for continuous endocrine monitoring and early interventions in chronic thyroid patients.
In addition, biomarkers such as thyroid hormone and thyroglobulin - both derived from liquid biopsy - have contributed to a non-invasive approach for staging progression of thyroid cancer and have shaped precision diagnosis in oncology and endocrinology. Nevertheless, the progress was hindered by challenges such as high cost of testing, lack of standardization of laboratory tests, and low availability of advanced thyroid testing, especially in the developing regions.
False negatives and false positives, patient lack of awareness for thyroid diseases, and regulatory intricacies hindered broad use. Nevertheless, with investment by firms in AI-powered diagnostic software, decentralized laboratory networks, and home-based cost-effective thyroid test kits, thyroid testing became cheaper, more accessible, and incorporated into regular endocrine screening programs.
Between 2025 and 2035, the market for thyroid function tests will experience revolutionary changes fuelled by AI-driven diagnostics, individualized thyroid biomarker profiling, and remote real-time thyroid monitoring. The convergence of molecular-level biomarker analysis, next-generation bio sensing technologies, and precision endocrinology will revolutionize early detection of thyroid disorders, disease monitoring, and therapeutic management.
The creation of AI-based thyroid function test interpretation platforms will facilitate automated, real-time risk stratification, combining thyroid hormone levels, genetic susceptibility, and environmental exposure variables to provide highly personalized endocrine health information. AI-powered predictive analytics models will identify subclinical hypothyroidism and hyperthyroidism prior to symptom onset, enabling proactive disease management and early pharmacological treatment.
With the use of nanotechnology-based biosensors, ultra-sensitive TSH, T4, and T3 assays will be available, improving early-stage thyroid dysfunction diagnosis. Using a one-step, point-of-care platform, the CRISPR-based biosensing technology will provide improved specificity in the detection of biomarkers, facilitating the quick identification of autoimmune thyroid indicators and thyroid hormone imbalance. Lab-on-a-chip platforms of the next generation will provide real-time blood biomarker analysis, enabling continuous monitoring of thyroid health via wearable devices.
The application of liquid biopsy methods for thyroid biomarkers will further enhance non-invasive thyroid cancer screening and disease monitoring. MicroRNA based thyroid function test kits will be developed by researchers to identify circulating miRNAs that are linked with autoimmune thyroid disease, thyroid nodules, and thyroid carcinoma progression.
The application of multi-omics biomarker panels consisting of genomic, proteomic, and metabolomics information will allow for extremely accurate patient-specific thyroid disorder diagnoses, informing individualized treatment plans for hormone replacement therapy and targeted thyroid cancer treatment.
AI-enabled home-based thyroid function testing kits will be the standard for routine endocrine health checks, as a result of the expansion of telemedicine and digital health technologies. Patients at risk of either hypothyroidism, hyperthyroidism, or thyroid cancer will then use AI-driven, real-time biomarker monitoring systems, transmitting continuous data to cloud-based endocrine monitoring centers.
These platforms will enable physicians to diagnose thyroid dysfunction earlier, make real-time adjustments to hormone therapy and remotely prevent complications of disease. In the way, a block chain-protected thyroid health data platform can provide better data protection, interoperability, and compliance in regulation on thyroid biomarkers monitoring-ensuring privacy and dependability in individualized endocrinology.
The future of thyroid function testing will also emphasize sustainability and cost reduction. AI-driven automated biomarker assay production, miniaturized diagnostic platforms, and disposable biosensors will lower manufacturing costs and environmental impact. The development of AI-assisted smart thyroid health management systems, automated diagnostic workflows, and decentralized thyroid screening networks will democratize access to high-precision thyroid diagnostics, ensuring global availability in both developed and emerging healthcare markets.
Market Shifts: A Comparative Analysis (2020 to 2024 vs. 2025 to 2035)
| Market Shift | 2020 to 2024 |
|---|---|
| Regulatory Landscape | Regulatory bodies expedited approval for AI-supported thyroid function tests, POC diagnostic panels, and multiplex biomarker tests. |
| Technological Advancements | Thyroid biomarker detection was enhanced using chemiluminescent immunoassays (CLIA), electrochemiluminescence assays (ECLIA), and AI-enabled biosensor platforms. |
| Industry Applications | Thyroid function testing became popular in the diagnosis of hypothyroidism, hyperthyroidism, autoimmune thyroid disease screening, and thyroid cancer testing. |
| Adoption of Smart Equipment | Hospitals, diagnostic laboratories, and POC testing facilities used automated TSH, T4, and T3 immunoassays, AI-driven diagnostic tools, and cloud-integrated data platforms. |
| Sustainability & Cost Efficiency | Firms concentrated on miniaturized ELISA-based assays, AI-optimized biomarker analysis, and cost-efficient high-sensitivity thyroid testing. |
| Data Analytics & Predictive Modelling | AI-optimized multi-biomarker analysis, real-time thyroid hormone fluctuation prediction, and biomarker standardization automation streamlined endocrine disorder diagnosis. |
| Production & Supply Chain Dynamics | Market impediments involved supply chain interference, cost hurdles for next-generation thyroid biomarker assays, and regulatory delays. |
| Market Growth Drivers | Growth was fuelled by growing thyroid disease incidence, escalating demand for quick biomarker diagnostics, and AI-enabled precision diagnostics. |
| Market Shift | 2025 to 2035 |
|---|---|
| Regulatory Landscape | AI-integrated thyroid diagnostics regulations, block chain-secured thyroid biomarker compliance, and CRISPR-based diagnostic standardization will shape future policies. |
| Technological Advancements | Quantum-powered biomarker detection, CRISPR-based thyroid biomarker screening, and nanotechnology-enhanced lab-on-a-chip diagnostics will revolutionize thyroid health monitoring. |
| Industry Applications | AI-assisted personalized thyroid health assessments, non-invasive liquid biopsy-based thyroid monitoring, and continuous real-time endocrine biomarker tracking will expand clinical applications. |
| Adoption of Smart Equipment | Home-based AI-powered thyroid biomarker tracking, real-time cloud-assisted endocrine health analytics, and decentralized biosensor-based thyroid function screening will redefine diagnostics. |
| Sustainability & Cost Efficiency | Self-powered biosensors, block chain-enabled endocrine health records, and AI-driven automated thyroid screening networks will improve sustainability and cost efficiency. |
| Data Analytics & Predictive Modelling | Quantum-assisted thyroid biomarker modelling, AI-powered adaptive endocrine health monitoring, and real-time digital twin simulations for thyroid disease prediction will enhance early detection and treatment. |
| Production & Supply Chain Dynamics | AI-driven biomarker supply chain optimization, decentralized thyroid diagnostic kit manufacturing, and block chain-enhanced thyroid data security will improve market accessibility. |
| Market Growth Drivers | The expansion of AI-powered precision endocrinology, real-time thyroid biomarker sensors, and sustainable endocrine disease management platforms will drive future market growth. |
The United States Thyroid Function Test market is growing steadily due to the increased incidence of thyroid disorders, rise in availability of the tests and use of home testing kits. As there are an estimated 20 million Americans with thyroid disease and more than 60% remain undiagnosed according to the American Thyroid Association, the case for universal thyroid function testing is strong. The market is driven by several factors, including the very high prevalence of hypothyroidism and hyperthyroidism in the elderly population and patients with autoimmune disorders, such as Hashimoto's thyroiditis and Graves' disease.
This has resulted in doctors recommending more frequent screenings. Consumerized genetic testing and telemedicine are expanding patient access to thyroid function test kits for measuring thyroid-stimulating hormone (TSH), free T4, and free T3 in the home setting. Furthermore, advances in technology relating to immunoassay-based thyroid laboratory tests enhance test performance and generate timely results, and high-throughput automated thyroid function analysers are being increasingly employed in laboratories.
| Country | CAGR (2025 to 2035) |
|---|---|
| USA | 5.1% |
Thyroid Function Test Market in the United Kingdom phenomenon Justification Growing prevalence of thyroid disorders, NHS-led screening initiatives, and advances in portable thyroid diagnostics drive the growth of the United Kingdom thyroid function test market.
It is estimated that 1 in 20 in the United Kingdom are living with a thyroid disorder, resulting in an increasing demand for routine TSH, T3 and T4 testing in primary care settings. Thyroid functionality tests can be afforded free through the National Health Service (NHS), and therefore promote early detection and management of diseases.
Moreover, increasing availability of digital health platforms & online diagnostic services is contributing to the growth of at-home thyroid test kits as people with thyroids prefer remote monitoring solutions. A growing incidence of iodine deficiency and autoimmune thyroid diseases is driving government-funded awareness campaigns, with the two promoting the adoption of screening and testing.
| Country | CAGR (2025 to 2035) |
|---|---|
| United Kingdom | 4.7% |
The Thyroid Function Test Market of European Union is estimated to have a high growth as there have been rising government awareness initiatives that screen for diseases of endocrine origin increasing funding in diagnostic research, as well as increasing aging population who have thyroid-related ailments.
The EU’s USD 5.23 billion Horizon Europe Program is backing innovation in all areas of healthcare diagnostics, including high-sensitivity and high-throughput assays for early detection of hypothyroidism and hyperthyroidism in thyroid function testing by providing funding to companies working on this area. Countries such as Germany, France, and Italy are at the forefront of thyroid function testing, with public health programs that promote the integration of routine thyroid screening in high-risk populations, such as pregnant women and the elderly.
The growing need for automated solutions and AI-after linked products for thyroid testing in turn is projected to expand the market by improving the accuracy of tests and lowering turnaround times and increasing accessibility in hospital and home-care settings.
| Country | CAGR (2025 to 2035) |
|---|---|
| European Union (EU) | 4.8% |
Immediate increase in the popularity of the thyroid function test in Japan is due to the increasing prevalence of thyroid disorder and healthcare reforms by the government, and improvements in automated thyroid testing technologies. Japan has a significantly aging population, with over 28% of its citizens aged 65 and above, resulting in a higher incidence of thyroid dysfunction, particularly hypothyroidism. The Japanese authorities are focusing on early detection measures and encouraging elderly patients and those with metabolic disorders to undergo periodic exams of their thyroids.
Advancements in AI technology, being utilized by diagnostic systems within hospital laboratories, are ensuring accuracy in thyroid tests, minimizing errors, and contributing to speedier diagnoses. The increasing use of wearable health tracking devices (such as smart wearables) that include thyroid function tracking is also a new trend in improving patient involvement in the longer-term management of thyroid disease.
| Country | CAGR (2025 to 2035) |
|---|---|
| Japan | 5.0% |
In case of South Korea, the Thyroid Function Test Market is growing at fastest pace owing to increasing cases of thyroid cancer, government-supported research on endocrine diseases, and augmenting inclination towards digital diagnostic platforms.
With one of the highest incidence of thyroid cancer in the world, South Korea underwent universal routine screening for thyroid function abnormalities. Its high-sensitivity TSH (Thyroid Stimulating Hormone) and FT4 (free Thyroxine) assays are developed as part of a USD 900 million investment by the South Korean Ministry of Health and Welfare in endocrine and cancer research.
AI and deep learning are playing an essential role in the digitization of diagnostic laboratories, facilitating accurate and efficient interpretation of thyroid function tests, minimizing human errors, and facilitating advanced and early detection rates.
Moreover, the growth of telemedicine and digital health platforms boosts the acceptance of home-based thyroid test kits, providing self-monitoring options for thyroid patients. Breaking new ground in early disease detection, particularly in high-risk patient groups.
| Country | CAGR (2025 to 2035) |
|---|---|
| South Korea | 5.1% |
The segments of the Thyroid Function Test market that have been covered in this report include the TSH (Thyroid-Stimulating Hormone) test and the FT4 (Free Thyroxine) test segments, as healthcare practitioners, diagnostic laboratories, and endocrinologists seek reliable, sensitive, and rapid diagnostic tests to assess thyroid health, detect abnormalities, and optimize treatment strategies.
These tests play a crucial role in the diagnosis of various thyroid disorders including hypothyroidism, hyperthyroidism, autoimmune thyroid disease, and metabolic disturbances, enabling early diagnosis, personalized treatment, and improved patient outcomes.
First empowering TSH test to dominate the market as the first-line screening measure for thyroid disease, considering the TSH test as a standard test in thyroid function testing, a first-line screening method for thyroid dysfunction disease, a tool for monitoring thyroid therapy, medication, and checking hormone imbalance, evaluation of metabolic disturbances with high sensitivity, accuracy, and important clinical value for early detection of thyroid dysfunction. TSH levels are used for diagnosing hypothyroidism and hyperthyroidism, as it is the body's mechanism in regulation with the other tests of thyroid function.
The rise in hypothyroidism and subclinical thyroid diseases has also bolstered the adoption of TSH testing, with clinicians seeking cost-effective, accurate diagnostic tools for routine screening, annual health examinations, and endocrine disease monitoring. TSH tests are now increasingly ordered from primary care providers, endocrinologists and gynaecologists to evaluate thyroid function of patients who present with weight gain or weight loss, fatigue, mood disorders and infertility issues.
The market is also experiencing an increase through an extensive adoption of TSH tests in prenatal and neonatal testing initiatives, where early detection of thyroid disorders can help to reduce the risk of developmental issues during pregnancy and early life, which is leading to better fetal growth health in the long run. Some studies showing a link between maternal hypothyroidism - if undiagnosed - and the development of mental impairment and birth defects have led obstetricians to make TSH testing universal.
Availability and convenience of in-home thyroid testing kits and telemedicine-based thyroid health monitoring have further increased patient access to these TSH testing options and made it possible for patients to access self-monitoring of their backdrop thyroid function and hormone levels and receive healthcare professional advice via an internet connection. TSH test results are combined with AI-based thyroid risk assessment in dedicated digital health platforms enabling the early detection, remote management of the disease, and personalized optimization of treatment.
The TSH testing which is of enormous clinical significance is affected by issues such as false negative in central hypothyroidism, variability of assay standardization and poor awareness related to thyroid screening guidelines. However, advancements in the form of high-sensitivity TSH assays, AI-based lab diagnostics, and combined thyroid hormone testing are improving diagnostic accuracy, reducing costs, and facilitating real-time monitoring of hormones, which will contribute to continued growth in the market.
The FT4 (Free Thyroxine) testing market is widely adopted in specialty testing, especially for differentiation of primary thyroid disorders, assessment of thyroid hormone replacement therapy, and to improve TSH test interpretation, as medical professionals strive for comprehensive evaluations of thyroid function. FT4 levels are obtained when free T4 levels are measured instead of total T4, meaning that you get a measurement of active unbound thyroxine instead.
The growing number of patients with autoimmune thyroid diseases, such as Hashimoto’s thyroiditis and Graves’ disease increases the need for FT4 testing as a patient requires an accurate measurement that balances free hormone concentration for confirmation of the thyroid dysfunction, treatment response, and to treat potential complications associated with these diseases. Tests for FT4 enable clinicians to substantively distinguish between overt and subclinical thyroid pathologies, which allows for timely intervention, remission, and therapeutic optimization.
In almost all of these scenarios, the addition of FT4 testing to TSH screening has improved the diagnosis of thyroid disorders, particularly among individuals deemed euthyroid on TSH screening, who are found to have abnormal thyroid hormone levels. Increasingly, healthcare providers rely on dual-thyroid function panels to identify non-classical thyroid dysfunctions, monitor for treatment of thyroidectomised patients, and evaluate the effectiveness of thyroid hormones replacement therapy.
In addition, in light of the increasing geriatric population and enhanced understanding of metabolic health risk, FT4 tests are, as a result, being utilized more widely because older adults have shown a greater susceptibility to thyroid dysfunction, cognitive impairment, and cardiovascular issues that have been associated with abnormalities in thyroid hormones. Routine FT4 screening is suggested by physicians amongst the older population, high-risk individuals, and patients in chronic disease management.
LTRs are highly relevant clinically but FT4 testing is hampered by issues with assay sensitivity, interference with biotin-containing supplements, and limited reimbursement by insurance companies in certain regions. Nonetheless, technological developments in immunoassay-based FT4 assays, AI-powered hormone analytics, alongside multiplexed endocrine disorder profile tests are rising the scale of accuracy, availability, and clinical decision-making of these tests, further substantiating an unrelenting need in FT4-driven thyroid diagnostics for the time being.
The hospitals and diagnostic laboratories segments represent the largest market drivers in the thyroid function test market, as healthcare providers prioritize comprehensive thyroid screening, endocrine disorder management, and preventive healthcare strategies.
Physicians are still the largest end users of thyroid function test kits, with thyroid hormone information very useful for preoperative evaluation, pregnancy follow-up and chronic disease management in hospitals. In contrast, hospitals perform comprehensive thyroid panel testing including TSH, FT4, FT3, and thyroid antibody screenings in a single case for a thorough assessment of endocrine health rather than point-of-care measurements.
The increase in hospital admission for thyroid-related conditions such as thyroid storm and myxoedema coma, both in patients with the diagnosis and those monitored post-thyroidectomy, has created an increasing demand for laboratory-based thyroid function testing, with rapid turnaround times for diagnostic testing and critical care decision making in the acute setting of patients requiring hospital admission.
Thyroid testing for cardiovascular disease, neuropsychiatric disorders, and metabolic conditions has been integrated into multidisciplinary care programs within hospital systems, where cardiologists, neurologists, and endocrinologists work together to evaluate the effects of thyroid hormone on disease.
The establishment of selective endocrine units and tertiary care hospitals offering state-of-the-art thyroid diagnostic testing equipment’s including automated immunoassay analysers, and high-throughput assays for hormones among others, and AI-based diagnostic systems, have better access and management of patients improve the precision and efficiency of testing.
Though such tests played a leading role in diagnosing thyroid disorders, hospital-based thyroid testing is plagued by issues such as high operating costs, short staff in laboratories, and inequity in testing at rural health centres. However, advances in hospital lab automation, point-of-care thyroid analysers, and cloud-connected diagnostic reporting systems are improving both the cost, efficiency, and scalability of tests, which is propelling continued expansion of the hospital-led thyroid test market.
The thyroid function test market has witnessed significant growth in this segment as diagnostic laboratories, reference labs and independent diagnostic centers have been providing cost-effective, high-volume thyroid panel test for preventive care and chronic disease management.
Just as direct-to-consumer (DTC) thyroid testing services have been trending, so has patient-demand for lab-based thyroid evaluations, allowing individuals to take their hormonal health into their own hands by screening for metabolic health and thyroid dysfunction long before clinical signs emerge.
An increase in either diagnosis demand or genetic predisposition could arguably explain the revolution in diagnostic laboratory efficiency brought on by the high-throughput immunoassay platforms and artificial intelligence-assisted thyroid biomarker analysis enabling the implementation of large-scale thyroid test panels with exceptional turnaround time, improved accuracy, and digital health integration.
Advancements in telemedicine and virtual health services have also played a significant role in enhancing access to thyroid testing, allowing patients to consult with specialists remotely and have their thyroid health assessed from the comfort of their homes, with samples collected for laboratory analysis when necessary.
Although diagnostic laboratory-based thyroid testing has flourished, it faces challenges, including pricing pressures from competing companies, barriers to insurance reimbursement for certain tests, and the ongoing need for standardization of assays. Trends such as evolving automated thyroid biomarker analysis, machine-learning-based prediction of endocrine disorders, and immediate reporting of actual diagnostic results will make laboratories more efficient, tests more affordable, and provide a more significant clinical impact, and in turn will maintain market demand for thyroid function testing.
The thyroid function test market is growing as a result of rising incidence of thyroid disease, technological developments in diagnostic technology, and point-of-care test adoption. Players emphasize high-sensitivity immunoassays, automated testing of thyroid hormones, and AI-based diagnostic solutions for improving early detection of disease, tailored treatment, and lab productivity. The market comprises global leaders and niche diagnostic companies, all of which play a role in the development of TSH, T3, T4, and thyroid antibody tests.
Market Share Analysis by Company
| Company Name | Estimated Market Share (%) |
|---|---|
| Abbott Laboratories | 12-17% |
| Siemens Healthineers AG | 10-14% |
| Roche Diagnostics | 9-13% |
| bioMérieux SA | 7-11% |
| Danaher Corporation (Beckman Coulter Diagnostics) | 5-9% |
| Other Companies (combined) | 45-55% |
| Company Name | Key Offerings/Activities |
|---|---|
| Abbott Laboratories | Develops high-sensitivity immunoassay-based thyroid function tests (ARCHITECT & Alinity platforms) for early diagnosis of hypothyroidism and hyperthyroidism. |
| Siemens Healthineers AG | Specializes in automated thyroid hormone assays, including TSH, Free T3, Free T4, and thyroid antibodies, enhancing clinical lab efficiency. |
| Roche Diagnostics | Manufactures high-throughput thyroid function test kits with AI-driven result analysis for hospital and point-of-care settings. |
| bioMérieux SA | Provides multiplex immunoassays and ELISA-based thyroid hormone detection tests, catering to endocrinology and metabolic disorder diagnostics. |
| Danaher Corporation (Beckman Coulter Diagnostics) | Offers integrated laboratory thyroid function testing solutions with real-time quality control and digital diagnostics. |
Key Company Insights
Abbott Laboratories (12-17%)
Abbott leads the market for thyroid function testing with high-sensitivity TSH, Free T3, and Free T4 assays to identify early thyroid disorder. It is a leader in point-of-care and automated diagnostics to improve clinical decision-making.
Siemens Healthineers AG (10-14%)
Siemens Healthineers excels at high-throughput thyroid function testing by combining automated lab workflows and artificial intelligence-powered thyroid hormone analysis. The company is advancing in personalized endocrine diagnostics.
Roche Diagnostics (9-13%)
Roche offers AI-driven thyroid function test solutions, focusing on high-precision immunoassays for hypothyroidism and hyperthyroidism screening. The company integrates cloud-based laboratory automation.
BioMérieux SA (7-11%)
BioMérieux provides multiplex and ELISA-based thyroid function tests, catering to endocrinology and metabolic disorder research. The company focuses on sensitive antibody testing for autoimmune thyroid diseases.
Danaher Corporation (Beckman Coulter Diagnostics) (5-9%)
Danaher manufactures automated thyroid function testing systems, with real-time quality control and high efficiency in laboratory diagnostics. The firm incorporates digital biomarker analysis for improved diagnostic precision.
Other Key Players (45-55% Combined)
Several diagnostic and biotechnology firms contribute to next-generation thyroid function testing, AI-based diagnostic tools, and multi-biomarker detection platforms. These include:
The overall market size for Thyroid Function Test Market was USD 1.8 Billion in 2025.
The Thyroid Function Test Market is expected to reach USD 2.9 Billion in 2035.
The demand for thyroid function tests will grow due to the rising prevalence of thyroid disorders, increasing awareness of early diagnosis, advancements in diagnostic technologies, and the expanding geriatric population, driving the need for efficient and accurate thyroid health assessments.
The top 5 countries which drives the development of Thyroid Function Test Market are USA, UK, Europe Union, Japan and South Korea.
TSH and FT4 Tests Drive Market to command significant share over the assessment period.
Epidemic Keratoconjunctivitis Treatment Market Overview – Growth, Trends & Forecast 2025 to 2035
Eosinophilia Therapeutics Market Insights – Trends & Forecast 2025 to 2035
Endometrial Ablation Market Analysis - Size, Share & Forecast 2025 to 2035
Endotracheal Tube Market - Growth & Demand Outlook 2025 to 2035
Encephalitis Treatment Market - Growth & Future Trends 2025 to 2035
Edward’s Syndrome Treatment Market – Growth & Future Prospects 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.