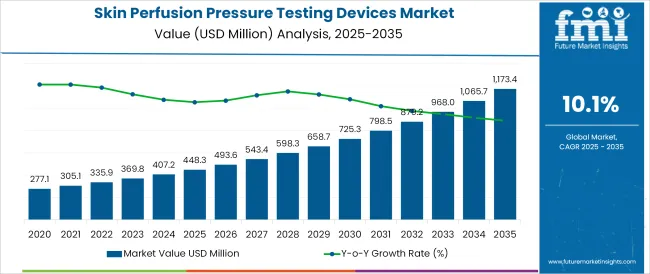
The Skin Perfusion Pressure Testing Devices Market is estimated to be valued at USD 448.3 million in 2025 and is projected to reach USD 1173.4 million by 2035, registering a compound annual growth rate (CAGR) of 10.1% over the forecast period.
The skin perfusion pressure (SPP) testing devices market is witnessing notable growth, fueled by increasing clinical awareness of peripheral artery disease and the need for accurate assessment of microcirculatory blood flow. Industry publications and clinical practice updates have emphasized the role of SPP testing in evaluating wound healing potential and guiding revascularization decisions.
Hospitals and vascular clinics have incorporated SPP testing into standard diagnostic protocols, particularly for diabetic foot ulcers and chronic limb ischemia management. Continuous technological advancements have led to the introduction of compact, user-friendly devices, enhancing accessibility across various healthcare settings.
Manufacturers have focused on developing non-invasive, real-time monitoring solutions, improving diagnostic accuracy and patient comfort. Future market expansion is expected through product innovation in portable and laser Doppler-based technologies and the rising demand for point-of-care diagnostic tools.
Segmental growth is anticipated to be driven by Laser Doppler Skin Perfusion Pressure Testing Devices, Portable Devices, and Hospitals as the primary end users, reflecting advancements in technology, ease of use, and the clinical necessity of these tools.
The market is segmented by Product, Modality, and End User and region. By Product, the market is divided into Laser Doppler Skin Perfusion Pressure Testing Devices, Photoplethysmography Skin Perfusion Pressure Testing Devices, Consumables, Pressure Cuff Controller, and Fibre Optic Probe. In terms of Modality, the market is classified into Portable Devices and Cart-based Devices.
Based on End User, the market is segmented into Hospitals, Ambulatory Surgical Centers, Wound Care Centers, and Vascular Laboratories. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
The Laser Doppler Skin Perfusion Pressure Testing Devices segment is projected to account for 42.5% of the market revenue in 2025, establishing itself as the leading product segment. Growth of this segment has been driven by the high sensitivity of laser Doppler technology in measuring microvascular blood flow, enabling accurate skin perfusion assessment.
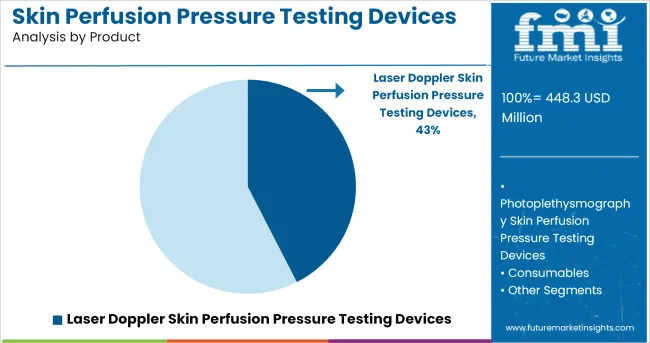
Hospitals and vascular specialists have preferred laser Doppler devices due to their ability to provide real-time, quantitative measurements, facilitating precise diagnosis and treatment planning. Continuous product enhancements have resulted in compact and ergonomic designs, improving user convenience and patient compliance.
Additionally, clinical publications have highlighted the role of laser Doppler in improving the prognosis of patients undergoing limb salvage procedures, reinforcing its clinical relevance. As demand increases for evidence-based vascular assessments, the Laser Doppler Skin Perfusion Pressure Testing Devices segment is expected to maintain its leadership through continuous technological evolution and expanding clinical adoption.
The Portable Devices segment is projected to contribute 56.3% of the skin perfusion pressure testing devices market revenue in 2025, solidifying its leadership in modality adoption. This segment’s growth has been driven by the demand for point-of-care vascular assessment tools, allowing clinicians to perform diagnostics in outpatient, home care, and remote clinical settings.
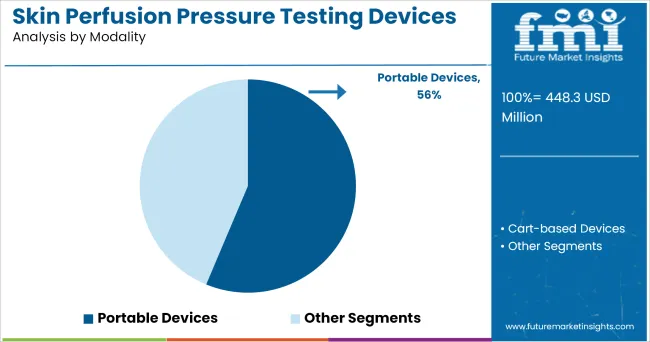
Portable SPP devices have been favored for their lightweight design, ease of transport, and rapid setup, enabling vascular assessments outside traditional hospital environments. Manufacturers have focused on developing user-friendly interfaces and wireless data transmission capabilities, supporting telehealth integration and remote monitoring.
Additionally, the portability of these devices has facilitated their use in wound care clinics and community health initiatives aimed at early detection of peripheral vascular disease. As healthcare delivery continues to shift toward decentralized and home-based care models, the Portable Devices segment is expected to retain its dominant position, supported by increasing demand for accessible diagnostic solutions.
The Hospitals segment is projected to account for 39.70% of the skin perfusion pressure testing devices market revenue in 2025, sustaining its leadership among end users. Growth of this segment has been driven by the clinical need for comprehensive vascular diagnostics in acute and chronic care settings.
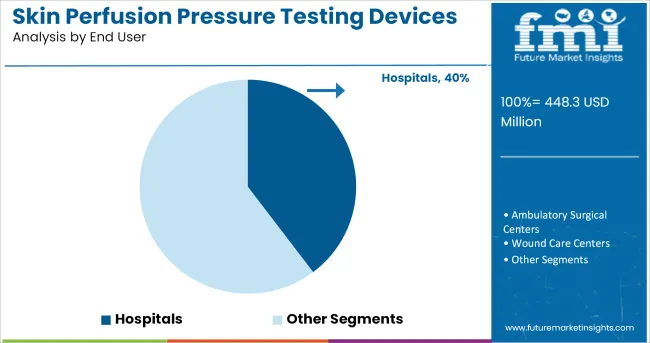
Hospitals have integrated SPP testing into multidisciplinary vascular care pathways, particularly in diabetic foot clinics, wound care centers, and vascular surgery units. Institutional procurement of advanced diagnostic equipment has prioritized devices with high diagnostic accuracy and patient safety, further boosting the adoption of SPP testing systems.
Hospitals have remained the preferred environment for managing complex vascular conditions, where immediate diagnostic and interventional capabilities are required. As reimbursement frameworks evolve to support preventative diagnostics and clinical guidelines recommend SPP testing for wound healing evaluation, the Hospitals segment is expected to remain the primary user base, reinforced by ongoing investments in advanced vascular diagnostics.
Peripheral artery disease (PAD) is a progressive disorder affecting more than 200 million people, worldwide. It has a significant impact on mortality in hemodialysis patients. Approximately 6.5 million people aged 40 years and older in the United States have PAD. The increasing PAD population has propelled the growth of the skin perfusion pressure testing market.
Skin perfusion pressure has become a potential tool for wound healing in patients with chronic limb ischemia. Compared to other non-invasive methods such as toe blood pressure and ankle-brachial index, skin perfusion pressure provides more accurate results, thus, its usage is gaining popularity among physicians.
This technique also remains a clinically relevant and objective tool of local perfusion in the setting of chronic limb ischemia, particularly among higher-risk patients with arterial calicifications and comorbidities that tend to microvascular disease. FMI expects global skin perfusion pressure testing devices market to grow at 4.5% CAGR through 2035.
Non-invasive procedures have continued to grow in popularity as compared to surgeries. The measurement of skin perfusion pressure by a laser doppler is a non-invasive method that is widely used by professionals. Due to high reproducibility, accuracy, specificity, and sensitivity, skin perfusion pressure testing has become one of the soundest methods.
For instance, non-invasive VMS-VASC system (Moor) Instruments), including the laser Doppler monitor moorVMS-LDF, low profile fibre optic probe (VP-SPP), and pressure cuff controller moorVMS-PRES have been specifically designed to aid micro vascular assessments in a variety of clinical applications and physiological research.
Laser dopplers have proven to be effective devices for the measurement of skin perfusion pressure. Laser dopplers are among the regularly utilized gadgets for the appraisal of skin perfusion pressure, they are seen as the ideal instrument for the location of peripheral artery disease as well as diagnosis of wound healing potential in limb ischemia patients.
Furthermore, the accuracy in a laser doppler is much higher than any other traditional method. The high accuracy, simplicity of operability and non-invasiveness feature of the devices are likely to fuel the utilization of laser dopplers by the physicians, boosting the demand of the skin perfusion testing devices.
Due to an increase in the need for assessment of wound healing in peripheral artery disease and limb ischemia, many manufacturers are seeking 510(k) Premarket Notification to FDA which demonstrates the device to be safe and effective as equivalent to a legally-marketed device
For example, Perimed Inc. seeks 510(k) Premarket Notification for PeriFlux System 5000, which is a laser doppler system consisting of main units and functional units
However, according to FDA Modernization Act, devices that fall under class I and II are exempted from premarket approval, thus, the time required for regulatory approval is reduced, which brings down the costs. The increasing FDA approvals attracts the end users, and this expected to boost the demand for skin perfusion pressure testing devices.
Skin pressure perfusion is the most advanced method for the assessment of wound healing in limb ischemia and peripheral artery diseases. This method requires highly trained or skilled professionals to operate the device.
Since it is fully-automated and provides quantitative evaluation of microcirculation, its operability requires skilled people, which as a result may limit the use of skin perfusion pressure testing devices.
Thus, reliability and reproducible measurement is highly dependent on a skilled technician or operator who interprets the results of skin perfusion pressure measurement. Lack of skilled professionals therefore can create significant challenges for the market.
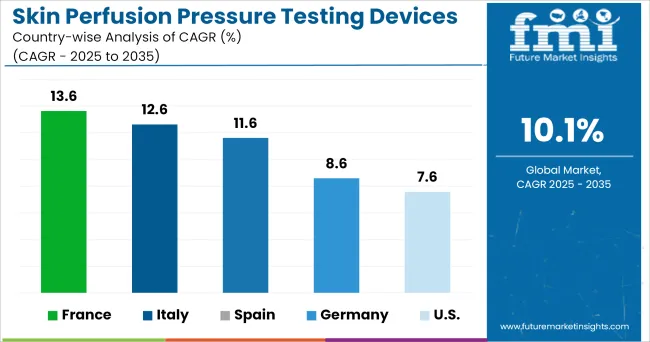
The healthcare system of the USA is well renowned for its advanced infrastructure and expenditure. The care regime of the care centers and hospitals are monitored by various regulatory rules and insurance policies. The massive hospital bills incurred other than the Medicare have been influencing the market in the country.
Government and regulatory bodies of the USA are adopting several cost-containment plans to reduce their healthcare burden. The shift from a volume to a value-based system is majorly driven by the various measures undertaken by providers, governments, biotechnology companies, and insurance payers.
Some of the measures are outcome-based profits, pricing, risk sharing, competitive tendering, and price control.
A value-based healthcare system will not only provide improved outcomes through integrated care pathways, but also levy pricing pressure on medical device companies, and this is likely to impact investments on the R&D of advanced medical treatment technologies.
Furthermore, due to the higher prevalence of critical limb ischemia and peripheral arterial disease in USA, several manufactures are conducting campaigns to raise general awareness among people related to these diseases.
This is likely to support the overall market growth in the country. For example, Amputee Coalition of America (NGO) launched a campaign in April 2020 to raise limb loss awareness among people.
The Federal Statistical Office has reported that healthcare spending in Germany rose by 4% in 2020 from 2020. In 2020, the country’s healthcare spending accounted for 11.7% of the GDP. Furthermore, health expenditure was expected to surge to 277.1 billion Euros by 2020, presenting a lucrative landscape for the expansion of the skin perfusion pressure testing devices.
Also presence of companies such as ELCAT GmbH is supporting the expansion in Germany. The company has been investing significantly in expansion efforts. Growth strategies adopted by these companies are expected to aid the expansion in Germany.
Since 2009, health insurance has been made mandatory for all citizens of the country. According to National Center for Biotechnology Information (NCBI), a total of 70 million people, which roughly covers 85% of the population are covered by statutory health insurance.
Another 11% population of Germany is covered by substitutive private health insurance. Conducive reimbursement policies and regulatory scenario are presenting attractive opportunities for growth.
Increasing disposable income can have significant impact in development of economy in emerging countries which results in affordability of proper healthcare treatment. China’s per capita disposable income reached (about 4,961 USA dollars) in 2024, more than double the level in 2010.
Potential for high healthcare insurance and rising disposable income will contribute to increase in affordability of healthcare services. This will also allow improved accessibility to expensive equipment for treatment of diseases. The market in China is expected to witness significant growth at a CAGR of around 5.4%.
Both chronic limb ischemia and peripheral artery disease impose serious threats to the population. Lack of awareness of diseases and lack of proper treatment leads to high prevalence of these diseases. Due to limited campaigning about the assessment of these diseases, there seems to occur less usage of these devices in hospitals in South Africa.
Moreover, poor healthcare facilities in some countries restrain the growth of the skin perfusion pressure testing market. Despite financial burden, government in South Africa is adopting various initiatives to spread create awareness among population, which is expected to enable steady growth in the market. The market in South Africa is expected to witness sluggish growth at a CAGR of just around 3.8%.
Laser dopplers are widely used for skin perfusion pressure testing. Due to easy operability and noninvasiveness, most physicians opt for laser dopplers. Since laser dopplers are costly, manufacturers provide reimbursements for such devices.
However, the use of laser dopplers is not only limited to the measurement of skin perfusion pressure. These can be used in a wide range of testing parameters such as toe blood pressure (TBP), post occlusive reactive hyperaemia (PORH), ankle brachial pressure index (ABPI), and others.
For example, Moor Instrument’s new moorVMS-VASC 2.0 monitoring system is designed to test pulse volume recording (PVR) and other parameters apart from skin perfusion pressure.
The portable devices segment will continue to grow and dominate the market during the forecast period. According to FMI, the segment is expected to hold around 73% of the market share, in terms of modality, by the end of 2035. A portable device is a wearable or hand-held device. This is designed for easy usage, to be able to move or carry it from one place to another, thus its ease of operability, makes the device most widely adopted devices in the market.
Hospitals have been dominating the market as key end users because it receives higher footfall of patients. Besides this, hospitals have more advanced infrastructure to support advance equipment. Backed by these factors, FMI forecasts hospitals to continue dominating the market and account for 34.7% of the overall share in 2024.
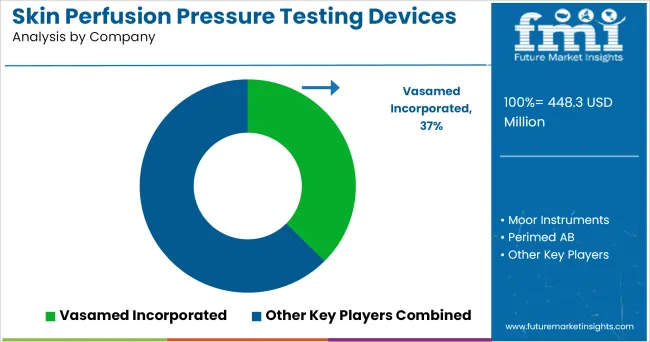
Most of the medical device companies are currently focusing on designing laser doppler since it has gain much popularity in assessment of wound healing.
For example, Moor Instruments has design moorVMS-VASC 2.0 laser doppler system. Moreover, the manufactures are focusing on getting stamp of approvals for their product and also entering into partnership and collaboration agreement to expand their product portfolio in different countries globally.
For instance, in April 2020, Perimed AB announced FDA approval for PeriFlux 6000.
Some of the leading companies operating in the market are:
| Attribute | Details |
|---|---|
| Forecast Period | 2025 to 2035 |
| Historical Data Available for | 2020 to 2020 |
| Market Analysis | USD Million for Value |
| Key Regions Covered | North America; Latin America; Europe; East Asia; South Asia; Oceania Middle East & Africa |
| Key Countries Covered | USA, Canada, Germany, UK, France, Italy, Spain, Poland, Russia, China, Japan, South Korea, India, Thailand, Malaysia, Indonesia, Australia, New Zealand, GCC Countries, Turkey, Northern Africa, South Africa |
| Key Segments Covered | Product Type, Modality, End User, and Region |
| Key Companies Profiled | Vasamed Incorporated; Moor Instruments; Perimed AB; ELCAT GmbH; ATYS Medical; Viasonix; Promed Group |
| Report Coverage | Market Forecast, Competition Intelligence, DROT Analysis, Market Dynamics and Challenges, Strategic Growth Initiatives |
| Customization & Pricing | Available upon Request |
The global skin perfusion pressure testing devices market is estimated to be valued at USD 448.3 million in 2025.
The market size for the skin perfusion pressure testing devices market is projected to reach USD 1,173.4 million by 2035.
The skin perfusion pressure testing devices market is expected to grow at a 10.1% CAGR between 2025 and 2035.
The key product types in skin perfusion pressure testing devices market are laser doppler skin perfusion pressure testing devices, photoplethysmography skin perfusion pressure testing devices, consumables, pressure cuff controller and fibre optic probe.
In terms of modality, portable devices segment to command 56.3% share in the skin perfusion pressure testing devices market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Skin Lightening Product Market Size and Share Forecast Outlook 2025 to 2035
Skincare Supplement Market Size and Share Forecast Outlook 2025 to 2035
Skin Tightening Device Market Size and Share Forecast Outlook 2025 to 2035
Skincare Oil Market Size and Share Forecast Outlook 2025 to 2035
Skin-Barrier Strengthening Phospholipids Market Size and Share Forecast Outlook 2025 to 2035
Skin Toner Market Size and Share Forecast Outlook 2025 to 2035
Skincare Nutritional Serum Market Size and Share Forecast Outlook 2025 to 2035
Skincare Products Market Size and Share Forecast Outlook 2025 to 2035
Skin Sensors Market Size, Growth, and Forecast for 2025 to 2035
Skin Grafting System Market Size and Share Forecast Outlook 2025 to 2035
Skincare Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Skincare Packaging Market Size, Share & Forecast 2025 to 2035
Skin Antiseptic Market - Demand, Growth & Forecast 2025 to 2035
Skin Bioactive Market Analysis - Trends, Growth & Forecast 2025 to 2035
Skin Replacement Market Growth - Trends & Forecast 2024 to 2034
Skin Tears Treatment Market Growth – Industry Outlook & Forecast 2024-2034
Skin Lightening Lip Balm Market Trends – Demand & Forecast 2024-2034
Skincare Industry in India – Trends & Growth Forecast 2024-2034
Global Skincare Treatment Market Analysis – Size, Share & Forecast 2024-2034
Skin Glossing Pencil Packaging Market

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA