Protein crystallization and crystallography market is expected to be a success in the future. Protein crystallization is paramount to studying the structures, interactions, and functions of proteins and hence, a key activity of pharmaceutical and biotechnological and academic research centers.
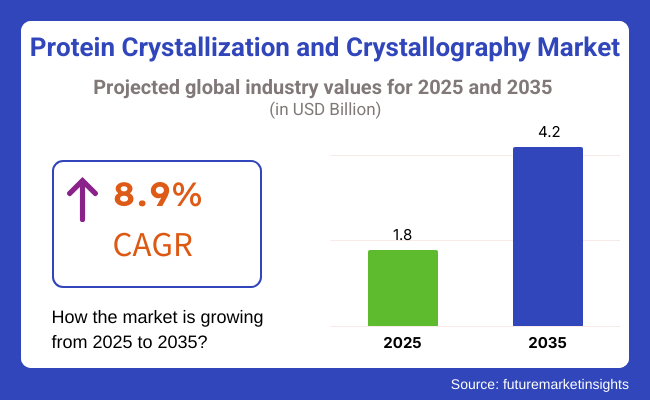
According to the new report published by Report Ocean, the market in Europe was USD 1.8 Billion in 2025 and is expected to be USD 4.2 Billion by 2035 growing at a healthy CAGR of 8.9% during the forecast period. The market for protein crystallization and crystallography is soaring, propelled by both the growing prevalence of chronic diseases and a demand for investigation of new biopharmaceutical compounds.
It has now been transformed through advances in X-ray crystallography, cryo-electron microscopy (cryo-EM), and new approaches to spectroscopic analysis. The integration of artificial intelligence (AI) and machine learning (ML) by structural biology in crystallization has also greatly improved the efficiency and accuracy of crystallization experiments.
Investment in biopharmaceutical R&D continues to inflament with the expansion of personalized medicine and increased consumption of structural genomics, making the protein crystallization and crystallography market primed for steady increases, creating immense opportunity for innovation and expansion in this field.
Explore FMI!
Book a free demo
North America dominates the protein crystallization and crystallography market, due to robust infrastructure for pharmaceutical R&D, established biotechnology sector and high investment by government in life sciences sector. The United States and Canada are top contributors, with premier academics, labs and pharmaceutical companies heavily funding structural biology."
In addition to that, the increasing interest in biologics drug development and precision medicine is increasing the use of crystallography methods to analyze the protein and design the drugs. In addition, agencies like the National Institutes of Health (NIH) and National Science Foundation (NSF) are continuously funding molecular biology and crystallography research projects.
Containing several top-notch biotech players, contract research organizations (CROs) and research collaborations, North America remains the main sales region for protein crystallization expansions.
Europe represents the second largest market for protein crystallization, but Germany, UK, and France are the leaders in the pace to the biotechnological innovations and structural biology research. This has led to widespread adoption protein crystallization techniques in the region through robust academic collaborations and pharmaceutical R&D investment.
Such growth in Europe is largely attributed to the increasing drug discovery programs and personalized medicine research along with the penetration of AI driven crystallization solutions. Moreover, public funding as well as initiatives from organizations like the European Molecular Biology Laboratory (EMBL) and Horizon Europe also sustain innovation in structural biology and crystallography.
Cryo-EM is also contributing to X-ray diffraction techniques synchrotron protein structural analysis which is also creating breakthroughs in new drug development and targeted therapies.
The Asia-Pacific market is anticipated to emerge as the fastest-growing market for protein crystallization and crystallography, due to the growing number of biopharmaceutical research activities, increasing investment in life sciences, and expanding healthcare infrastructure in the region. China, India, Japan, and South Korea are investing heavily in protein and structural biology projects to facilitate drug and disease research efforts.
The growth of its own biotechnology industry, along with government programs that promote pharmaceutical innovation, have made China a destination for applications of crystallography. North and South America and India all further develop their pharmaceutical Research and development investment and contract research services, improving research of protein crystallization.
These countries have been stepping up their prowess in spectroscopy techniques, cryo-EM technology, and bioinformatics-driven crystallography and are solidifying their presence in the international protein research scene.
The Asian-Pacific protein crystallization market is likely to witness lucrative growth over the future years, owing to the rising count of biopharmaceutical startups, academic collaborations, as well as government-led life sciences programs.
Challenges
Opportunities
The protein crystallization & crystallography market was valued over USD 489 Million in 2020 and is expected to grow to over USD 807 Million by ND 2024, at a CAGR of 9% between 2020 & 2024. By solving the three-dimensional structures of biomolecules through protein crystallization strategies, scientists and pharmaceutical industry may easily optimize various procedures such as in the discovery of targeted therapeutics, engineering of useful enzymes, manipulation of clinically relevant vaccines.
X-ray crystallography, cryo-electron microscopy (cryo-EM), and more recently nuclear magnetic resonance (NMR) spectroscopy enlarged in scale, providing high-throughput structural determination of proteins, antibodies, and more drug targets, thus accelerating the development of next-generation biologics and small-molecule drugs. These efforts built on the previous investment of USA and EU regulatory agencies (USA Food and Drug Administration (FDA), European Medicines Agency (EMA) and National Institutes of Health (NIH)) funding structural genomics initiatives to study disease-related proteins.
The present study demonstrated the usefulness of automated protein crystallization platforms, microfluidics approaches for crystallization, and artificial intelligence (AI) based structure prediction models in enhancing the efficiency of drug target identification and lead optimization research being performed by pharmaceutical and biotech companies. High-throughput screening (HTS) micro crystallography enabled the ability to quickly analyze protein structures, which supported the goal of speeding up the drug discovery process and preclinical validations.
Significant improvements in accuracy of structure determination of proteins have come from advances in technology, including free-electron lasers (XFELs), synchrotron-based X-ray diffraction methods, as well as state-of-the-art cryo-EM methods.
In recent years, AI-based computational modelling and machine learning-based protein folding simulations (e.g., DeepMind’s Alpha Fold) have revolutionized the landscape by allowing researchers to predict protein-ligand interactions without additional experimental crystallization steps. The technology led to rapid advances in the field, driving the development of targeted therapies for cancer, neurodegenerative disease, and infectious disease.
Although progress was made quickly, the market did not expand due to issues like challenges in crystallizing membrane proteins, availability so costly equipment, and limited access to synchrotron radiation facilities.
Moreover, the considerable expertise required for protein purification, stability testing and crystallization meant very few smaller research institutions were able to adopt this practice widely. Nevertheless, with the advent of automated crystallization screening systems, AI-driven predictive models, and microfluidics-based nano-crystallization methods from various companies, the protein structure analysis will be more accessible, propelling sustained growth in the market.
AI-based molecular modelling, quantum-enhanced structure prediction, and nanotechnology-driven crystallization techniques are ready to revolutionise the protein crystallization and crystallography market from 2025 to 2035. Combining AI-driven protein folding simulations with new cryo-EM and XFEL technologies will enable researchers to determine protein structures at atomic resolution and in real-time, while greatly decreasing dependence on traditional X-ray crystallography methods.
Quantum computing will facilitate faster screening of drug candidates, while increasing the accuracy of predicting bimolecular interactions through molecular docking. Data driven by AI-enhanced bioinformatics pipelines will facilitate the automated optimization of crystallization conditions, resulting in soluble, stable and diffracting-quality proteins. AI-powered high-throughput crystallization robotics of the next generation is set to increase the rate of protein crystal screening automation and minimize human involvement to improve reproducibility of high-resolution protein structure determination.
Introduction of 3D bioprinted systems for protein crystallization using synthetic bio material scaffolds stably incubated to promote organized growth of protein crystals. If you can investigate this in lab-on-a-chip microfluidic devices, you can perform multiplex crystallization experiments from tiny volumes, and-belying the myth of crystallization-crystallization will be a very efficient process used in structural biology, drug discovery, and enzyme engineering.
This nanoparticle-assisted crystallization will become a transformative method allowing stabilization of membrane proteins, intrinsically-disordered proteins, and complex protein-protein assemblies that have long resisted crystallization.
In order to gather these multiple conformational states and ensemble data, AI-driven hybrid modelling strategies will combine cryo-EM, NMR, and crystallography datasets and thus perform multi-modal protein structures determination with greater accuracy. AI-driven synthetic biology platforms aid rational protein engineering, by providing machine learning solutions to the problem of optimizing crystallization conditions for drug target identification or rational design of novel enzymes.
The increasing adoption of personalized medicine and precision therapeutics will further fuel demand for these AI-assisted drug discovery platforms, enhancing structure-based drug design (SBDD) and fragment-based drug discovery (FBDD) strategies.
This will also see sustainability and cost reduction as a driving narrative in the shaping of the market. Miniaturized crystallization platforms, advanced automation, and open-access AI-powered structure prediction tools will democratize structural analysis of proteins, ensuring small biotech companies and academic institutions can access state-of-the-art crystallization technologies. In the long term, it is expected that AI-augmented cloud-based structural bioinformatics databases in a collaborative research framework will promote real-time data sharing and expedite discoveries in structural biology.
Market Shifts: A Comparative Analysis (2020 to 2024 vs. 2025 to 2035)
| Market Shift | 2020 to 2024 |
|---|---|
| Regulatory Landscape | Increased government and funding agency support helped to underpin efforts to accomplish structural genomics giving rise to investments into X-ray crystallography and cryo-EM facilities. |
| Technological Advancements | Protein structure determination improved with AI-driven simulations, cryo-EM, XFELs, and high-throughput crystallization techniques. |
| Industry Applications | Pharmaceutical companies used X-ray crystallography and cryo-EM to develop targeted cancer therapies, enzyme inhibitors, and monoclonal antibodies. |
| Adoption of Smart Equipment | Automated crystallization systems, microfluidics-driven nano-crystallization, and AI-based structure modelling improved research efficiency. |
| Sustainability & Cost Efficiency | The market faced high operational costs, limited synchrotron facility access, and labour-intensive crystallization screening challenges. |
| Data Analytics & Predictive Modelling | AI-assisted molecular docking and ligand-binding prediction models enhanced drug discovery pipelines. |
| Production & Supply Chain Dynamics | Challenges in protein purification, stability, and crystallization reproducibility slowed the translation of structural data into drug development. |
| Market Growth Drivers | Improvements in cryo-EM, automated crystallization robotics, and AI-based structure prediction models have driven market expansion. |
| Market Shift | 2025 to 2035 |
|---|---|
| Regulatory Landscape | It will incorporate AI-based protein structure prediction systems, quantum-supported crystallographic devices, and real-time structure assessment guidelines that will regulate industry adherence. |
| Technological Advancements | Quantum computing-driven molecular docking, AI-powered hybrid modelling, and nanoparticle-assisted protein crystallization will redefine structural biology. |
| Industry Applications | AI-enhanced personalized medicine, precision drug design, and nanotechnology-driven bimolecular engineering will expand the role of protein crystallography in healthcare. |
| Adoption of Smart Equipment | AI-driven hybrid modelling, 3D bioprinting of protein crystals, and self-learning bioinformatics platforms will accelerate high-throughput structural biology research. |
| Sustainability & Cost Efficiency | Cloud-based AI platforms, decentralized bioinformatics resources, and automated protein structure prediction algorithms will reduce costs and improve accessibility. |
| Data Analytics & Predictive Modelling | Quantum-enhanced protein-ligand interaction simulations, edge computing-based crystallization analytics, and AI-driven bimolecular structural databases will revolutionize structure-based drug design. |
| Production & Supply Chain Dynamics | Automated AI-driven protein folding simulations, synthetic biology-assisted protein design, and scalable quantum molecular simulations will streamline drug discovery pipelines. |
| Market Growth Drivers | Fueling this growth will be quantum-assisted structure-based drug design, AI-powered protein engineering, and nanotechnology-enhanced bimolecular research. |
High demand for drug discovery and investments are being made on structural biology research, which drive the growth of the market as well. To that end, the USA invests more than any other nation in biopharmaceutical innovation by analyzing protein structure for the discovery of new drugs.
Increasing government grants for research in biomedical and structural biology is one of the major market drivers. More sophisticated crystallization methods, X-ray diffraction (XRD), and cryo-electron microscopy (cryo-EM) are more accessible, with over USD 7 billion of contributions from the National Science Foundation (NSF) and the National Institutes of Health (NIH) to structural biology research.
Another key factor is the growing use of protein crystallography in drug development. With over 50% of drug discovery protocols hinging on the determination of protein structures, larger pharma players like Pfizer, Merck, and Amgen are investing tons of money on automated protein crystallization platforms.
Use of artificial intelligence (AI) and machine learning (ML) is slowly creeping into the discipline, which, in turn, is transforming it, enabling us to rapidly predict structures of proteins and thereby improving the rate of crystallization success.
In addition, next-generation crystallization methods are being developed at large-scale research institutions such as Harvard, MIT, and Stanford, also helping to increase the market.
| Country | CAGR (2025 to 2035) |
|---|---|
| USA | 9.2% |
The Initiatives of the UK Government's Life Sciences Initiatives Will Fuel Strong Growth through Academia/Biotech Collaborations, Demand for Protein Structure-Based Drug Discovery, and Rising Demand.
London, and more recently through the availability of world-class research institutions (e.g., University of Oxford, University of Cambridge, and Francis Crick Institute) dedicated to advancing macromolecular crystallography and structural bioinformatics.
Pharmaceutical firms are incorporating these systems into protein crystallization in response to the mounting demand from application fields like precision medicine and genomics research, pointing to more accurate protein structure determination in shorter timeframes.
UK-based CROs and biotech start-ups have also been at the forefront of improving next-generation cryo-EM and X-ray diffraction.
| Country | CAGR (2025 to 2035) |
|---|---|
| UK | 8.7% |
The European Union Protein Crystallization and Crystallography market is expanding on the back of robust government initiatives in favor of life sciences, enhanced collaboration between the pharmaceutical industry and academia, and the development of automated crystallography technology.
The EU Horizon Europe Program, with a budget of €5 billion for structural biology and drug discovery research, is driving the innovation in protein crystallization technologies, X-ray diffraction, and cryo-EM techniques.
Germany, France, and the Netherlands are where drug discovery and structural genomics are in the forefront, with top research institutions such as EMBL (European Molecular Biology Laboratory) and Max Planck Institute at the forefront of molecular structure examination.
The growing demand for AI-enabled crystallization and bioinformatics integration is enabling high-throughput protein-ligand interaction screening, speeding up the pace of early-stage drug development.
Besides, the increasing collaborations of pharma giants such as Roche, Sanofi, and Novartis with EU-based R&D centers are driving next-generation protein crystallization platforms.
| Country | CAGR (2025 to 2035) |
|---|---|
| European Union (EU) | 8.9% |
Japanese protein crystallization and crystallography market is expanding with the advancement in structural biology research, biopharmaceutical discovery funding, and growing use of AI in protein research. Japan possesses a robust research ecosystem led by top organizations such as RIKEN, Kyoto University, and the University of Tokyo driving innovative macromolecular crystallization research.
The Japanese government has committed ¥300 billion (USD 2.5 billion) in funding to biomedical and drug discovery research, much of which will significantly expand the funding for X-ray crystallography, cryo-EM, and neutron diffraction methods.
The other major driver is Japan's sizzling pharma sector, dominated by Takeda, Astellas, and Daiichi Sankyo. Industry players are investing in AI-driven crystallization methods to optimize drug development pipelines.
Japan is also a leader in protein crystallization with synchrotrons, with institutions such as the Spring-8 Synchrotron Radiation Facility at the forefront of structural genomics and protein-ligand interaction studies.
| Country | CAGR (2025 to 2035) |
|---|---|
| Japan | 9.1% |
The protein crystallography and crystallization market of South Korea is growing at a fast pace attributed to increased investment in structural genomics, increased application in drug discovery, and increased application of AI and automation in crystallography.
A faster convergence among drug companies, biotech companies, and universities is fueling macromolecular crystallization and drug-target interaction studies at a rapid rate. POSTECH (Pohang University of Science and Technology) and KAIST (Korea Advanced Institute of Science and Technology) are leaders in advancing cryo-EM and X-ray crystallography.
High-capacity protein crystallization technologies are also on rising demand with Celltrion and Samsung Biologics and others' biologics and biosimilar production and development.
Additionally, the combination of protein modeling and cloud-computational chemistry with artificial intelligence is also enhancing the efficiency of protein crystallization pipelines.
| Country | CAGR (2025 to 2035) |
|---|---|
| South Korea | 9.2% |
Cryo-electron microscopy (Cryo-EM) and X-ray crystallography segment leads the protein crystallization and crystallography market with pharmaceuticals, biotechnology firms, and research institutions using high-resolution structural determination technology to mechanize molecular biology research, protein engineering, and drug discovery. The technology is of paramount significance in protein interaction, structure, and function expertise to ensure improved drug design, biomolecular characterization, and targeted therapeutic discovery.
X-ray crystallography is becoming the gold-standard tool of protein-ligand interaction research, structural biology, and molecular-level drug design, delivering high-resolution three-dimensional biomolecular structure. Researchers use this technique to reveal atomic-level information on receptor-ligand binding interactions, enzymatic processes, and protein conformations to facilitate rational drug design and precision medicine innovation.
Pharmaceutical and biopharmaceutical companies increasingly turn towards X-ray crystallography to assist with drug discovery, vaccine engineering, and antibody optimization because of the ability to yield precise structural knowledge required for precision drug design. Structure-based drug design (SBDD) draws on the details of X-ray crystallography in a bid to predict best-fit binding sites, increase drug affinity of binding, enhance pharmacokinetics in efforts towards effective lead optimization and limiting rates of failure within clinical trials.
Academic and research groups also benefit tremendously from X-ray crystallography as researchers investigate protein-protein interaction, enzyme catalysis, and molecular dynamics. Protein folding diseases, gene mutations, and metabolic pathways are investigated by structural biologists to learn about the mechanism of the disease in more detail along with therapeutic response.
With the increase in spreading pathogenic infections and newly identified emerging viruses, researchers apply X-ray crystallography to research viral proteins, vaccine structural antigens, and immunity, further speeding next-generation vaccine design and monoclonal antibody discovery.
All its glory and precision notwithstanding, X-ray crystallography is marred by problems of protein crystallization, weeks- or month-long sample preparation, and intractability of very flexible or membrane-bound proteins. Despite this, advances in automated crystallization robots, synchrotron radiation sources, and artificial intelligence-based structure prediction tools are driving data rate of acquisition, resolution accuracy, and crystallization success, hence setting the stage for future market expansion.
Cryo-electron microscopy (Cryo-EM) has been accepted in the market for decades, particularly in structural virology, nucleic acid-protein complexes, and membrane proteins, since it represents the ability of near-atomic resolution without crystalline samples. Compared to X-ray crystallography with highly ordered protein crystals, Cryo-EM is capable of potentially imaging native biomolecular structures in physiological conditions, which enabled dynamic conformational analysis and mechanistic studies..
The biopharmaceutical industry increasingly uses Cryo-EM for target-directed drug discovery, protein engineering, and structure-based antibody discovery because it can provide visualization of molecular interaction, allosteric sites, and dynamic conformational change. Pharmaceutical industries use Cryo-EM structural information to rationalize biologics, RNA drugs, and small-molecule inhibitors for enhancing therapeutic activity and specificity of treatment.
The Cryo-EM has also revolutionized the virology field, and scientists have been tracing the viral capsid, spike protein, and immune evasion at high resolution. Scientists globally employed Cryo-EM protocols to resolve the SARS-CoV-2 spike protein structure that led to the creation of mRNA vaccination and discovery of monoclonal antibodies.
The neurodegenerative disease and structural genomics communities come to rely on Cryo-EM more to investigate amyloid fibrils, protein aggregates, and intracellular assemblies to discover Alzheimer's disease, Parkinson's disease, and prion disease mechanisms. With its ability to visualize protein misfolding and aggregation processes in real time, Cryo-EM facilitates therapeutic design in age-related disease and neurobiology.
In spite of being of such high promise, Cryo-EM is beset by problems such as high-end equipment, sophisticated data analysis, and requirement of specialized computing centers. Nevertheless, advances in AI-assisted image reconstruction, automated vitrification of samples, and next-generation direct electron detectors are driving resolution higher, throughput higher, and cost of operation lower, and holding out the prospect of greater use of Cryo-EM in research in structural biology and drug discovery.
Consumables such as capillaries, microplates, sample grids, and coverslips perform a very significant function in protein crystallization experiments through the provision of accurate sample handling, best crystallization conditions, and reproducible data collection. Researchers apply disposable crystallization equipment to facilitate vapor diffusion, microbatch, and lipidic cubic phase (LCP) crystallization methods, thereby allowing effective screening and scale-up of protein crystals.
The advent of automated protein crystallization has fueled the need for high-end consumables since scientists need precision-engineered plates, nanometer-thick film membranes, and nanoliter-volume crystallization chips in order to achieve efficient crystallization and minimize the use of samples. The next-generation microfluidic-based consumables provide real-time monitoring of crystallization, faster screening, and high-throughput structural biology experiments.
Growth in complexity of membrane protein crystallization dictated the need for specialty consumables like lipidic cubic phase (LCP) crystallization plates, glass sandwich plates, and mesophase sample holders whose use enhances proteins that are recalcitrant to crystallization to exhibit higher stability and diffracting quality.
Although necessary in protein structure studies, consumables are subject to high cost, contamination danger, and instability in quality of manufacture. Improvements in recent precision microfabrication, automation-compatible crystallization equipment, and ultra-low volume screening plate technology, however, are enhancing reproducibility, cost-effectiveness, and ease of access to experiments and assuring long-term market demand for protein crystallization consumables.
Crystallization screening solutions and reagent kits have gained strong market penetration, particularly in pharmaceutical drug discovery, structural genomics, and research institutions, since researchers must possess optimal buffer conditions, stabilizing chemicals, and crystallization additives in order to maximize protein structure determination rates.
High-throughput crystallization screening tools, including commercially available crystallization screens, pH gradient sets, and precipitant libraries, allow researchers to rapidly identify the optimal crystallization conditions, improving protein stability and crystal growth reproducibility. Pre-formulated reagent sets eliminate trial-and-error experiments, reducing experimental variability and speeding protein structure analysis pipelines.
The growth of fragment-based drug discovery (FBDD) has further boosted the demand for tailor-made crystallization reagent kits, as pharmaceutical firms use optimized screening buffers, cross-linking agents, and cryoprotectants to stabilize protein-drug complexes for structural studies.
The growing utility of AI-aided crystallization optimization has created machine-learning-based reagent selection algorithms that predict optimal buffer conditions, temperature settings, and additive formulations for better crystallization efficiency and diffraction quality.
Though extensively used, reagent kits and screening solutions are plagued by issues of low stability, batch-to-batch inconsistency, and high purchase price. Next-generation crystallization buffer formulations, microfluidic-based reagent mixing technologies, and AI-based screening approaches are improving reproducibility, experimental success rates, and overall cost, guaranteeing long-term market expansion.
The protein crystallization and crystallography market is expanding with increasing need for structural biology studies, drug discovery, and advanced X-ray diffraction (XRD) technology. Organizations focus on automated crystallization platforms, high-throughput screening technologies, and artificial intelligence-based structural analysis to enhance protein structure determination, pharmaceutical R&D, and biotechnology applications.. The market consists of worldwide leaders and specialized producers, each adding technological progress in crystallization methods, diffraction analysis, and 3D molecular modeling.
Market Share Analysis by Company
| Company Name | Estimated Market Share (%) |
|---|---|
| Rigaku Corporation | 12-17% |
| Bruker Corporation | 10-14% |
| Thermo Fisher Scientific, Inc. | 9-13% |
| Hampton Research | 7-11% |
| Molecular Dimensions Ltd. | 5-9% |
| Other Companies (combined) | 45-55% |
| Company Name | Key Offerings/Activities |
|---|---|
| Rigaku Corporation | Develops X-ray diffraction (XRD) systems, automated protein crystallization platforms, and high-resolution imaging tools for structural biology research. |
| Bruker Corporation | Specializes in high-throughput X-ray crystallography, cryo-electron microscopy (Cryo-EM), and NMR spectroscopy for protein structure analysis. |
| Thermo Fisher Scientific, Inc. | Offers automated crystallization systems, reagents, and AI-driven data analysis platforms for protein crystallization studies. |
| Hampton Research | Provides customized crystallization screens, high-purity reagents, and robotic crystallization solutions for drug discovery applications. |
| Molecular Dimensions Ltd. | Focuses on optimized protein crystallization plates, cryo-protection solutions, and crystallography screening kits for laboratory research. |
Key Company Insights
Rigaku Corporation (12-17%)
Rigaku is dominant net protein crystal growth and crystallography market due to its state-of-the-art X-ray diffraction systems, an automatic, advanced crystallization tools The company integrates AI-guided imaging analysis with real-time crystallization monitoring to enable biopharma R&D
Bruker Corporation (10-14%)
Bruker is a leader in next-generation X-ray crystallography and Cryo-electron microscopy (Cryo-EM), enabling drug discoverers to get more structural information for new drugs. Efficiency in Biotech and Pharmaceutical Research with Bruker's High-Throughput Crystallization Solutions
Thermo Fisher Scientific, Inc. (9-13%)
ThermoFisher supplies integrated platforms for crystallization and high-resolution imaging instrumentation for the structural analysis of biological macromolecules. The company specializes in automation screening crystallization and artificial intelligence-driven molecular structure prediction.
Hampton Research (7-11%)
Hampton Research specializes in protein crystallization screens, purified reagents, and custom crystallization solutions. Hampton Research supports academic, pharmaceutical, and biotechnology laboratories engaged in structural biology research.
Molecular Dimensions Ltd. (5-9%)
Molecular Dimensions have a specialty in crystallization and consumables, screening plates, and cryo-protection solutions, helping customers optimize workflow for protein structure determination.
Other Key Players (45-55% Combined)
Several manufacturers contribute to advanced crystallization technologies, high-precision imaging systems, and AI-driven protein structure modeling. These include:
The overall market size for protein crystallization and crystallography market was USD 1.8 Billion in 2025.
The protein crystallization and crystallography market is expected to reach USD 4.2 Billion in 2035.
The demand for protein crystallization and crystallography is expected to rise due to increasing applications in drug discovery, structural biology, and biopharmaceutical research, where understanding protein structures is essential. Advancements in ai-driven crystallography, automation, and high-throughput screening will further accelerate adoption, enhancing precision in targeted therapy development and disease research.
The top 5 countries which drives the development of protein crystallization and crystallography market are USA, UK, Europe Union, Japan and South Korea.
Consumables and Reagent Kits to command significant share over the assessment period.
Specialty Medical Chairs Market Trends - Size, Growth & Forecast 2025 to 2035
Surgical Drapes Market Overview - Growth, Demand & Forecast 2025 to 2035
Super Resolution Microscope Market Insights - Size, Share & Forecast 2025 to 2035
Portal Hypertension Management Market Trends - Size, Growth & Forecast 2025 to 2035
Precocious Puberty Treatment Market Overview – Growth, Trends & Demand Forecast 2025 to 2035
Pleural Diseases Therapeutics Market – Drug Trends & Future Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.