The prostate-specific antigen testing industry is valued at USD 7.9 billion in 2025. As per FMI's analysis, the industry will grow at a CAGR of 12.0% and reach USD 24.6 billion by 2035.
FMI analysis concluded that the industry of PSA testing experienced steady development in 2024 as a result of the accelerating adoption of newer diagnostic equipment and the growing application of screening across the globe.
North America led adoption, with doctors incorporating AI-driven PSA interpretation solutions for improved risk assessment. European regulatory authorities authorized new ultra-sensitive assays in sync, additionally accelerating test accuracy as well as the rate of early detection.
FMI believes 2025 will mark a turning point as healthcare policies increasingly prioritize early prostate cancer detection. Strategic partnerships among biotech companies and diagnostic labs will improve accessibility, especially in developing economies. More investments in point-of-care PSA testing will fuel demand, diminishing the dependency on centralized laboratory diagnostics.
Post-2025, developments in biomarker science are expected to narrow test specificity and reduce false negatives and positives. Increased reimbursement coverage in major industries, including the United States and Germany, will also facilitate high-scale adoption. Asia-Pacific will, however, experience strong growth due to increasing awareness and upgraded healthcare infrastructure.
Industry Forecast Table
| Metric | Value |
|---|---|
| Industry Size (2025E) | USD 7.9 billion |
| Industry Value (2035F) | USD 24.6 billion |
| CAGR (2025 to 2035) | 12.0% |
The prostate-specific antigen (PSA) testing industry is poised for long-term growth, fueled by advances in diagnostic technology and greater focus on early detection of prostate cancer. FMI research found that doctors, testing labs, and biotech companies making accurate tests will benefit from the growing demand, while traditional lab tests might be challenged by fast, on-site testing developments.
FMI believes that as insurers and governments broaden screening coverage, access will increase, setting the stage for long-term steady growth in the industry.
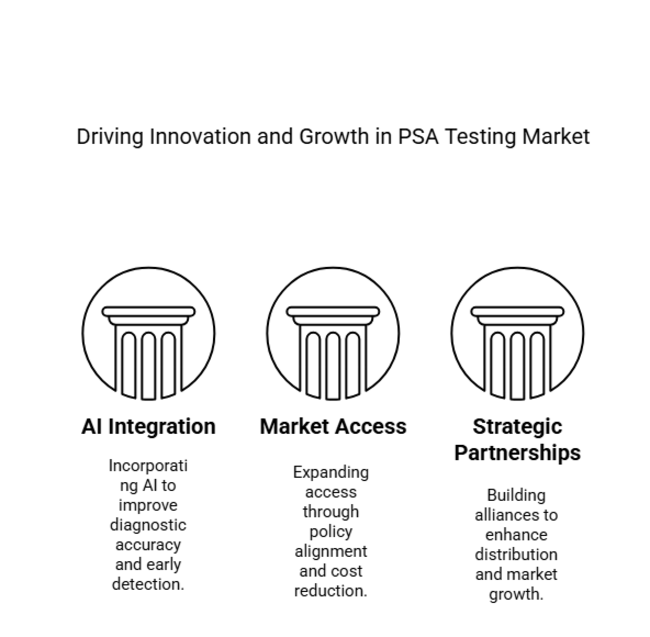
Invest in AI-Driven Diagnostic Upgrades
Executive management must lead the incorporation of artificial intelligence within PSA testing to drive greater accuracy, eliminate false positives, and grow early detection rates. Collaboration with AI-specialty biotech companies will be integral to preserving competitiveness.
Increase Industry Access Through Policy and Price Realignment
Aligning with changing healthcare policies and broadening reimbursement coverage will be essential to penetration in the industry. Firms must collaborate with regulatory bodies and insurers to increase access and lower the cost of PSA testing.
Enhance distribution and strategic partnerships
FMI believes that partnership with hospitals, laboratory testing facilities, and telehealth providers will fuel growth in the industry. Spending on international distribution channels and point-of-care testing platforms will promote long-term industry leadership.
| Risk | Probability & Impact |
|---|---|
| Regulatory Delays in New Test Approvals | High Probability - High Impact |
| Competition from Emerging Non-PSA Biomarkers | Medium Probability - High Impact |
| Reimbursement Policy Uncertainty | High Probability - Medium Impact |
| Priority | Immediate Action |
|---|---|
| AI Integration in PSA Testing | Partner with AI-focused biotech firms to enhance diagnostic accuracy |
| Reimbursement Expansion | Engage with insurers and policymakers to secure broader test coverage |
| Point-of-Care Testing Growth | Develop and launch rapid PSA test kits for decentralized diagnostics |
To stay ahead, executives must accelerate investment in AI-powered PSA diagnostics and point-of-care testing to capitalize on shifting healthcare trends. FMI's analysis shows that changes in regulations and more funding options create a chance to increase use, but competition from other biomarkers could challenge long-term success.
FMI opines that aligning R&D with emerging precision medicine initiatives and forging strategic partnerships with insurers and healthcare providers will be critical to sustaining industry leadership.
Over the next 12 months, the roadmap should prioritize securing regulatory approvals for next-gen tests, expanding distribution in high-growth regions, and leveraging AI to enhance diagnostic accuracy, ensuring a competitive edge in the evolving PSA testing landscape.
Regional Variance
High Variance
Material Preferences
Regional Differences
Shared Challenges
89% reported higher operating costs with PSA testing from reagent price increases.
Regional Differences
Manufacturers
Hospitals & Labs
Worldwide Synergy
75% of PSA test manufacturers are investing in AI and multi-marker development.
Divergence
Keystone Variances
Strategic Insights
Leaders in this industry would be those that can localize PSA test pricing by region, embed technology, and maneuver through regulation.
| Countries | Regulatory Impact & Mandatory Certifications |
|---|---|
| United States | The FDA controls PSA tests using the Clinical Laboratory Improvement Amendments (CLIA) and a strict approval process for new tests, which makes it harder for them to enter the industry. Overall, CMS sets reimbursement policies that dictate whether tests can be afforded. |
| United Kingdom | In UKCA marking, PSA testing is regulated under MHRA; NICE sets the guidelines for screening, but no national screening program is currently present, restraining mass adoption. |
| France | PSA tests are governed by ANSM and European IVDR regulations, and national health insurance reimburses PSA screening only in selected conditions, generating access barriers. |
| Germany | As part of In-Vitro Diagnostic Regulation (IVDR), PSA testing is reimbursable only if certain medical conditions are reached, which limits the applicability of routine screenings. |
| Italy | The approval of PSA tests is also subject to the oversight of AIFA, while the differing reimbursement policies for the same test, based on regions, lead to discrepancies in the test being adopted by different healthcare facilities. |
| South Korea | PSA tests are regulated by the Ministry of Food and Drug Safety (MFDS). The government promotes early detection of cancer, but national screening coverage is very limited. |
| Japan | The Pharmaceuticals and Medical Devices Agency (PMDA) mandates stringent validation for PSA tests; no nationwide prostate cancer screening program exists. |
| China | In China, PSA diagnostics are regulated by the NMPA. Due to a focus on cost-effective solutions, government policies tend to restrict the adoption of high-end PSA tests. |
| Australia-NZ | Australian Therapeutic Goods Administration (TGA) and New Zealand Medsafe; PSA tests are available in both countries, but PSA screening is not included in routine government-funded screening programs. |
| India | PSA test approvals are regulated by the Central Drugs Standard Control Organization (CDSCO) in India, but there are currently no formalized national guidelines regarding the inclusion of PSA testing as part of routine screening, leading to varying accessibility of the tests. |
Based on test type, chemiluminescent immunoassays (CLIA) are anticipated to be the largest industry, fueled by the enhanced sensitivity of this method, suitability for automation, and rapid turnaround potentially reducing diagnostic delays and improving patient outcomes during 2025 to 2035.
With laboratories and diagnostic centers increasingly driving toward high-throughput methods, CLIA has become the gold standard for PSA testing due to its improved accuracy and reduced variability compared to traditional ELISA or lateral flow assays.
The analysis study indicates that the adoption rate will be driven by the increasing focus on early-stage detection of prostate cancer and constant developments in CLIA platforms in the years to come. In light of these propellers, the CLIA segment is anticipated to grow at a rate of approximately 13.1% during 2025 to 2035, which surpasses the overall industry CAGR of 12.0%.
Serum represents the most lucrative sample type segment in the global prostate cancer diagnostics industry in 2025 and 2035 owing to the familiar adoption of serum samples as the conventional sample source for PSA tests. As they are derived from serum, these tests are stable, consistent, and yield higher-quality results than tests based on whole blood or plasma.
Advancements in serum-based biomarker detection along with a higher preference for automated immunoassays will drive the segment growth, according to FMI analysis. Furthermore, the ongoing regulatory approvals of serum-based PSA tests only fortify the clinical utility of PSA. Considering these factors, the serum segment is expected to grow at a CAGR of 12.5% over the forecast period.
Diagnostic laboratories are expected to remain key intermediaries from 2025 to 2035 from 2025 to 2035 in terms of end-user segment, as patients increasingly favor lab-based tests over hospital-based tests, and investments in computerized PSA screening platforms will continue on an uptrend to support commercial-scale deployment.
According to FMI analysis, diagnostic labs outperform point-of-care settings in terms of test accuracy and scalability and are therefore the widely adopted option among oncologists and urologists.
Collaborations between labs and AI-powered diagnostic companies are improving test accuracy and decreasing turnaround times. Based on these motivating factors, the Diagnostic Laboratories segment is expected to be the fastest-growing segment at a CAGR of nearly 13.3% throughout the forecast period.
From 2025 to 2035, projections indicate a CAGR of 12.3% for the PSA testing industry in the United States. The United States: The country continues to be a major growth area owing to high adoption of AI-powered diagnostics, growing awareness about prostate cancer screening, and advanced healthcare infrastructure.
FDA certification begets rigorous validation of each test, but lengthy approval processes delay new technologies entering the industry.
Reimbursement policies have been found to impact accessibility, with expanded coverage correlating with increases in test volumes, according to FMI analysis of Medicare and private insurer reimbursement policies. FMI predicts partnerships with AI-based diagnostics companies are the key to industry penetration.
Estimates indicate that the PSA testing industry in the UK will grow at a CAGR of 11.1% between 2025 and 2035. Rising public awareness and healthcare investments drive the industry, but the absence of a national screening program restricts its widespread adoption. According to FMI analysis, the UKCA marking and MHRA oversight are in place to govern PSA testing, serving to assure quality standards.
FMI opines that alignment with the NHS's strategy and inclusion in more general cancer screening programs could drive growth. Incrementally improving test adoption will also help further transition the industry to biomarker-based prostate cancer. These qualities make this industry attractive to firms with a strong focus on innovation.
The PSA testing industry size in France is expected to grow at an annual rate of 10.5% between 2025 and 2035. Requirements such as ANSM and IVDR, which direct high-quality standard diagnostic assays, substantiate the industry. The national health insurance system partially covers PSA tests, but selective reimbursement policies prevent wider access, according to FMI analysis.
FMI sees expansion driven by increasing demand for early-stage cancer detection and biomarker-based diagnostics. With the new approvals for manufacturers in France following strict rules, companies in this field need to make sure they meet these approval standards, especially for accurate PSA (prostate-specific antigen) tests along with genetic risk profiling.
Forecasts indicate a CAGR of 11.8% for the PA industry in Germany from 2025 to 2035. The country has a compartmentalized cancer screening system, but the PSA test is reimbursed under strict conditions, meaning not all men are automatically tested.
IVDR compliance creates higher entry barriers for new PSA assays, according to a recent FMI analysis, but Germany is the leader in precision diagnostics. FMI predicts that multi-marker prostate cancer screening methods that factor in PSA along with genetic and molecular biomarkers will take off. Collaboration with hospitals and research institutes will be crucial in driving test accessibility and industry expansion.
From 2025 to 2035, the Italy PSA testing industry is estimated to grow at a compound annual growth rate (CAGR) of 10.2%. Italy’s PSA testing framework involves region-specific payer collaboration, with discrepancies in reimbursement policies and oversight by AIFA. Despite increasing early detection initiatives, inconsistent insurance coverage continues to limit access to PSA tests, according to FMI analysis.
The availability of tests will increase due to rising investments in healthcare and growing adoption of digital pathology (Source S64692). It is necessary for industry players to collaborate with regional healthcare authorities to make reimbursement policies more efficient and raise physician-patient awareness about advanced options for real-time diagnosis.
Projections indicate a CAGR of 11.5% for the PSA testing industry in South Korea from 2025 to 2035. Although the country's health system prioritizes preventive diagnostics, limited health insurance coverage hinders the widespread use of PSA testing.
MFDS regulations dominate the industry, with extensive clinical validation needed prior to acceptance of new tests, according to an FMI analysis. Both the increasing availability of point-of-care PSA testing and the inclusion of AI-powered diagnostics, according to FMI, are driving growing adoption. South Korea, meanwhile, will see a steady industry for companies that specialize in affordability and fast diagnostics.
In Japan, the PSA testing realm is anticipated to grow at a CAGR of 10.7% over the period of 2025 and 2035. Despite a highly advanced healthcare system, basic screening with PSA is not common and is not part of a national screening program for cultural reasons.
The FMI analysis revealed that PMDA regulations create a challenging pathway for the approval of new tests, resulting in a significant slowdown in industry access. According to FMI, an increasing interest in precision diagnostics and AI-based PSA testing will promote adoption. The industry in Japan will mainly benefit from strategic partnerships with research institutions and hospitals.
The PSA sector in China is projected to grow at a CAGR of 13.2% between 2025 and 2035, making it one of the world's fastest-growing industries. According to FMI analysis, high cancer prevalence, increasing healthcare expenditure, and large-scale diagnostic programs funded by the government will be supporting the industry growth.
In contrast, NMPA regulations favor cost-effective solutions that watch PSA test adoption with advanced AI. Localized manufacturing, pricing adjustments, and partnerships with state governments will remain key to success in this landscape, opines FMI. Increasing incorporation of PSA testing among rural healthcare platforms will boost industry penetration further.
Moreover, projections indicate a CAGR of 10.9% for the PSA testing industry in Australia and New Zealand between 2025 and 2035. This advantage stems from an established healthcare infrastructure industry, although it lacks government-funded routine PSA screening programs.
While TGA (Australia) and Medsafe (NZ) regulations guarantee high diagnostic quality in FMI analysis, assimilation is still physician-driven. FMI believes that coupling PSA tests with expanded men’s health programs and digital health platforms will drive growth. A focus on at-home and telehealth-based testing solutions represents an important cross-sell opportunity.
The PSA testing industry in India is projected to grow at a CAGR of 13.5% for the period extending from 2025 to 2035, highlighting the fast-paced growth in this industry. FMI's analysis also revealed that the absence of national screening guidelines and low awareness levels impede early detection efforts.
But demand is being driven by increased healthcare spending, urbanization, and rising incidence rates of cancer. PSA testing remains cost-sensitive, and industry expansion is being driven by government-funded early detection programs, according to FMI. This nascent industry has high potential; focusing on collaborative efforts with diagnostic laboratories and insurers for accessibility and affordability will be key.
Major players of the Prostate-Specific Antigen (PSA) Testing industry are fighting with pricing plans, technological development, strategic collaboration, and geographic expansion. Companies are investing intensely in AI-powered diagnostics, high-sensitivity immunoassays, and automation to improve the precision and speed of tests, according to FMI analysis.
Mergers and acquisitions are restructuring the competitive landscape as major companies acquire niche biotech startups to make their offerings robust. Moreover, hospital partnerships and diagnostic chain partnerships are creating an expanding industry size.
Pricing policies differ among firms; some offer cost-effective ELISA kits, while others focus on high-end chemiluminescence assays (CLIA) for high-throughput laboratories. Moves into emerging nations continue to remain a priority area.
Abbott Laboratories
Share: ~20-25%
A dominant leader in diagnostics, Abbott has an excellent position within PSA testing from its Alinity immunoassay systems to its worldwide network of distributors.
Roche Diagnostics
Share: ~18-22%
Roche is a strong industry leader with its Elecsys PSA tests, taking advantage of its automated cobas systems and emphasis on high-throughput laboratories.
Siemens Healthineers
Share: ~15-18%
Siemens is competitive with its Advia Centaur and Atellica PSA assays, highlighting accuracy and integration with lab automation.
Danaher Corporation (Beckman Coulter)
Share: ~12-15%
Beckman Coulter's Access PSA tests and DxI immunoassay systems play their role in maintaining its stable industry share, especially in Europe and North America.
Thermo Fisher Scientific
Share: ~8-12%
Thermo Fisher's Brahms PSA assays and ImmunoDiagnostics business target specialized laboratories, emphasizing precision and clinical utility.
Other Players (Bio-Rad, Fujirebio, etc.)
Share: ~10-15% combined
Smaller competitors such as Fujirebio (with its Lumipulse G PSA tests) and Bio-Rad focus on smaller niches, such as research and new industries.
The industry is segmented into immunoassays, elisa kits, lateral flow assays, chemiluminescent immunoassays (clia), radioimmunoassay, point-of-care testing kits, cassette/cards, strips.
The industry is segmented into whole blood, serum, plasma.
The industry is segmented into hospitals, diagnostics laboratories, specialty clinics, research institutes, others.
The industry is studied across North America, Latin America, Europe, South Asia, East Asia, Oceania, Middle East and Africa (MEA)
Rising incidence of prostate cancer, heightened early detection awareness, improvements in high-sensitivity immunoassays, and are driving increased demand for PSA testing solutions.
The industry will see robust growth, underpinned by technological advancements, AI-driven diagnostic solutions, and rising laboratory uptake of chemiluminescent immunoassays (CLIA).
Major providers are Xiamen Biotime Biotechnology Co. Ltd, HWTAi, OptiBio Co., Ltd, Jiangsu MicroDiag Biomedicine Technology Co., Ltd, Beijing Hotgen Biotechn Co., Ltd, Humasis, Accuquik™ Test Kits, CTK Biotech, Inc, INTEC, XIAMEN BOSON BIOTECH CO., LTD, AccuBioTech Co., Ltd, Bio-Rad Laboratories, Inc, Accuquik Test Kits. It also includes OPKO Health, Inc, bioMérieux SA, Beckman Coulter, Inc, Abbott, Siemens Healthineers, DiaSorin, F. Hoffmann-La Roche Ltd, Mediwatch (LABORIE), BodiTech, Bristol-Myers Squibb Company, GE Healthcare, Endocare, GlaxoSmithKline, Anixa Biosciences, Ortho Clinical, Fujirebio, Roche Diagnostics, Danaher Corporation, Thermo Fisher Scientific.
Chemiluminescent immunoassays (CLIA) are anticipated to dominate driven by superior accuracy, automatization capabilities, and increased use in diagnostic laboratories.
The prostate-specific antigen (PSA) test industry is forecasted to reach USD 24.6 billion by the year 2035 due to increasing screening efforts and newer diagnostic technologies.
Table 01: Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By Test Type
Table 02: Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033 By Sample Type
Table 03: Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By End User
Table 04: Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By Region
Table 05: North America Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By Country
Table 06: North America Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By Test Type
Table 07: North America Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By Sample Type
Table 08: North America Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By End User
Table 09: Latin America Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By Country
Table 10: Latin America Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By Test Type
Table 11: Latin America Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By Sample Type
Table 12: Latin America Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By End User
Table 13: Europe Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By Country
Table 14: Europe Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By Test Type
Table 15: Europe Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033 By Sample Type
Table 16: Europe Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By End User
Table 17: East Asia Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By Country
Table 18: East Asia Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By Test Type
Table 19: East Asia Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By Sample Type
Table 20: East Asia Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By End User
Table 21: South Asia Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By Country
Table 22: South Asia Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By Test Type
Table 23: South Asia Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By Sample Type
Table 24: South Asia Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By End User
Table 25: Oceania Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By Country
Table 26: Oceania Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By Test Type
Table 27: Oceania Market Analysis 2018 to 2022 and Forecast 2023 to 2033 By Sample Type
Table 28: Oceania Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By End User
Table 29: Middle East & Africa Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By Country
Table 30: Middle East & Africa Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By Test Type
Table 31: Middle East & Africa Market Analysis 2018 to 2022 and Forecast 2023 to 2033 By Sample Type
Table 32: Middle East & Africa Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, By End User
Figure 01: Market Value (US$ Million) Analysis, 2018 to 2022
Figure 02: Market Forecast & Y-O-Y Growth, 2023 to 2033
Figure 03: Market Absolute $ Opportunity (US$ Million) Analysis, 2022 to 2033
Figure 04: Market Value Share (%) Analysis 2023 and 2033, By Test Type
Figure 05: Market Y-O-Y Growth (%) Analysis 2022 to 2033, By Test Type
Figure 06: Market Attractiveness Analysis 2023 to 2033, By Test Type
Figure 07: Market Value Share (%) Analysis 2023 and 2033, By Sample Type
Figure 08: Market Y-O-Y Growth (%) Analysis 2022 to 2033, By Sample Type
Figure 09: Market Attractiveness Analysis 2023 to 2033, By Sample Type
Figure 10: Market Value Share (%) Analysis 2023 and 2033, By End User
Figure 11: Market Y-O-Y Growth (%) Analysis 2022 to 2033, By End User
Figure 12: Market Attractiveness Analysis 2023 to 2033, By End User
Figure 13: Market Value Share (%) Analysis 2023 and 2033, By Region
Figure 14: Market Y-O-Y Growth (%) Analysis 2022 to 2033, By Region
Figure 15: Market Attractiveness Analysis 2023 to 2033, By Region
Figure 16: North America Market Value (US$ Million) Analysis, 2018 to 2022
Figure 17: North America Market Value (US$ Million) Forecast, 2023 to 2033
Figure 18: North America Market Value Share, By Test Type (2023 E)
Figure 19: North America Market Value Share, By Sample Type (2023 E)
Figure 20: North America Market Value Share, By End User (2023 E)
Figure 21: North America Market Value Share, By Country (2023 E)
Figure 22: North America Market Attractiveness Analysis by Test Type, 2023 to 2033
Figure 23: North America Market Attractiveness Analysis by Sample Type, 2023 to 2033
Figure 24: North America Market Attractiveness Analysis by End User, 2023 to 2033
Figure 25: North America Market Attractiveness Analysis by Country, 2023 to 2033
Figure 26: USA Market Value Proportion Analysis, 2022
Figure 27: Global Vs. USA Growth Comparison, 2022 to 2033
Figure 28: USA Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 29: USA Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 30: USA Market Share Analysis (%) By End User, 2022 & 2033
Figure 31: Canada Market Value Proportion Analysis, 2022
Figure 32: Global Vs. Canada. Growth Comparison, 2022 to 2033
Figure 33: Canada Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 34: Canada Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 35: Canada Market Share Analysis (%) By End User, 2022 & 2033
Figure 36: Latin America Market Value (US$ Million) Analysis, 2018 to 2022
Figure 37: Latin America Market Value (US$ Million) Forecast, 2023 to 2033
Figure 38: Latin America Market Value Share, By Test Type (2023 E)
Figure 39: Latin America Market Value Share, By Sample Type (2023 E)
Figure 40: Latin America Market Value Share, By End User (2023 E)
Figure 41: Latin America Market Value Share, By Country (2023 E)
Figure 42: Latin America Market Attractiveness Analysis by Test Type, 2023 to 2033
Figure 43: Latin America Market Attractiveness Analysis by Sample Type, 2023 to 2033
Figure 44: Latin America Market Attractiveness Analysis by End User, 2023 to 2033
Figure 45: Latin America Market Attractiveness Analysis by Country, 2023 to 2033
Figure 46: Mexico Market Value Proportion Analysis, 2022
Figure 47: Global Vs Mexico Growth Comparison, 2022 to 2033
Figure 48: Mexico Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 49: Mexico Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 50: Mexico Market Share Analysis (%) By End User, 2022 & 2033
Figure 51: Brazil Market Value Proportion Analysis, 2022
Figure 52: Global Vs. Brazil. Growth Comparison, 2022 to 2033
Figure 53: Brazil Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 54: Brazil Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 55: Brazil Market Share Analysis (%) By End User, 2022 & 2033
Figure 56: Argentina Market Value Proportion Analysis, 2022
Figure 57: Global Vs Argentina Growth Comparison, 2022 to 2033
Figure 58: Argentina Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 59: Argentina Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 60: Argentina Market Share Analysis (%) By End User, 2022 & 2033
Figure 61: Europe Market Value (US$ Million) Analysis, 2018 to 2022
Figure 62: Europe Market Value (US$ Million) Forecast, 2023 to 2033
Figure 63: Europe Market Value Share, By Test Type (2023 E)
Figure 64: Europe Market Value Share, By Sample Type (2023 E)
Figure 65: Europe Market Value Share, By End User (2023 E)
Figure 66: Europe Market Value Share, By Country (2023 E)
Figure 67: Europe Market Attractiveness Analysis by Test Type, 2023 to 2033
Figure 68: Europe Market Attractiveness Analysis by Sample Type, 2023 to 2033
Figure 69: Europe Market Attractiveness Analysis by End User, 2023 to 2033
Figure 70: Europe Market Attractiveness Analysis by Country, 2023 to 2033
Figure 71: UK Market Value Proportion Analysis, 2022
Figure 72: Global Vs. UK Growth Comparison, 2022 to 2033
Figure 73: UK Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 74: UK Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 75: UK Market Share Analysis (%) By End User, 2022 & 2033
Figure 76: Germany Market Value Proportion Analysis, 2022
Figure 77: Global Vs. Germany Growth Comparison, 2022 to 2033
Figure 78: Germany Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 79: Germany Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 80: Germany Market Share Analysis (%) By End User, 2022 & 2033
Figure 81: Italy Market Value Proportion Analysis, 2022
Figure 82: Global Vs. Italy Growth Comparison, 2022 to 2033
Figure 83: Italy Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 84: Italy Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 85: Italy Market Share Analysis (%) By End User, 2022 & 2033
Figure 86: France Market Value Proportion Analysis, 2022
Figure 87: Global Vs France Growth Comparison, 2022 to 2033
Figure 88: France Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 89: France Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 90: France Market Share Analysis (%) By End User, 2022 & 2033
Figure 91: Spain Market Value Proportion Analysis, 2022
Figure 92: Global Vs Spain Growth Comparison, 2022 to 2033
Figure 93: Spain Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 94: Spain Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 95: Spain Market Share Analysis (%) By End User, 2022 & 2033
Figure 96: Russia Market Value Proportion Analysis, 2022
Figure 97: Global Vs Russia Growth Comparison, 2022 to 2033
Figure 98: Russia Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 99: Russia Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 100: Russia Market Share Analysis (%) By End User, 2022 & 2033
Figure 101: Benelux Market Value Proportion Analysis, 2022
Figure 102: Global Vs Benelux Growth Comparison, 2022 to 2033
Figure 103: Benelux Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 104: Benelux Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 105: Benelux Market Share Analysis (%) By End User, 2022 & 2033
Figure 106: East Asia Market Value (US$ Million) Analysis, 2018 to 2022
Figure 107: East Asia Market Value (US$ Million) Forecast, 2023 to 2033
Figure 108: East Asia Market Value Share, By Test Type (2023 E)
Figure 109: East Asia Market Value Share, By Sample Type (2023 E)
Figure 110: East Asia Market Value Share, By End User (2023 E)
Figure 111: East Asia Market Value Share, By Country (2023 E)
Figure 112: East Asia Market Attractiveness Analysis by Test Type, 2023 to 2033
Figure 113: East Asia Market Attractiveness Analysis by Sample Type, 2023 to 2033
Figure 114: East Asia Market Attractiveness Analysis by End User, 2023 to 2033
Figure 115: East Asia Market Attractiveness Analysis by Country, 2023 to 2033
Figure 116: China Market Value Proportion Analysis, 2022
Figure 117: Global Vs. China Growth Comparison, 2022 to 2033
Figure 118: China Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 119: China Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 120: China Market Share Analysis (%) By End User, 2022 & 2033
Figure 121: Japan Market Value Proportion Analysis, 2022
Figure 122: Global Vs. Japan Growth Comparison, 2022 to 2033
Figure 123: Japan Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 124: Japan Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 125: Japan Market Share Analysis (%) By End User, 2022 & 2033
Figure 126: South Korea Market Value Proportion Analysis, 2022
Figure 127: Global Vs South Korea Growth Comparison, 2022 to 2033
Figure 128: South Korea Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 129: South Korea Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 130: South Korea Market Share Analysis (%) By End User, 2022 & 2033
Figure 131: South Asia Market Value (US$ Million) Analysis, 2018 to 2022
Figure 132: South Asia Market Value (US$ Million) Forecast, 2023 to 2033
Figure 133: South Asia Market Value Share, By Test Type (2023 E)
Figure 134: South Asia Market Value Share, By Sample Type (2023 E)
Figure 135: South Asia Market Value Share, By End User (2023 E)
Figure 136: South Asia Market Value Share, By Country (2023 E)
Figure 137: South Asia Market Attractiveness Analysis by Test Type, 2023 to 2033
Figure 138: South Asia Market Attractiveness Analysis by Sample Type, 2023 to 2033
Figure 139: South Asia Market Attractiveness Analysis by End User, 2023 to 2033
Figure 140: South Asia Market Attractiveness Analysis by Country, 2023 to 2033
Figure 141: India Market Value Proportion Analysis, 2022
Figure 142: Global Vs. India Growth Comparison, 2022 to 2033
Figure 143: India Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 144: India Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 145: India Market Share Analysis (%) By End User, 2022 & 2033
Figure 146: Indonesia Market Value Proportion Analysis, 2022
Figure 147: Global Vs. Indonesia Growth Comparison, 2022 to 2033
Figure 148: Indonesia Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 149: Indonesia Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 150: Indonesia Market Share Analysis (%) By End User, 2022 & 2033
Figure 151: Malaysia Market Value Proportion Analysis, 2022
Figure 152: Global Vs. Malaysia Growth Comparison, 2022 to 2033
Figure 153: Malaysia Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 154: Malaysia Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 155: Malaysia Market Share Analysis (%) By End User, 2022 & 2033
Figure 156: Thailand Market Value Proportion Analysis, 2022
Figure 157: Global Vs. Thailand Growth Comparison, 2022 to 2033
Figure 158: Thailand Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 159: Thailand Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 160: Thailand Market Share Analysis (%) By End User, 2022 & 2033
Figure 161: Oceania Market Value (US$ Million) Analysis, 2018 to 2022
Figure 162: Oceania Market Value (US$ Million) Forecast, 2023 to 2033
Figure 163: Oceania Market Value Share, By Test Type (2023 E)
Figure 164: Oceania Market Value Share, By Sample Type (2023 E)
Figure 165: Oceania Market Value Share, By End User (2023 E)
Figure 166: Oceania Market Value Share, By Country (2023 E)
Figure 167: Oceania Market Attractiveness Analysis by Test Type, 2023 to 2033
Figure 168: Oceania Market Attractiveness Analysis by Sample Type, 2023 to 2033
Figure 169: Oceania Market Attractiveness Analysis by End User, 2023 to 2033
Figure 170: Oceania Market Attractiveness Analysis by Country, 2023 to 2033
Figure 171: Australia Market Value Proportion Analysis, 2022
Figure 172: Global Vs. Australia Growth Comparison, 2022 to 2033
Figure 173: Australia Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 174: Australia Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 175: Australia Market Share Analysis (%) By End User, 2022 & 2033
Figure 176: New Zealand Market Value Proportion Analysis, 2022
Figure 177: Global Vs New Zealand Growth Comparison, 2022 to 2033
Figure 178: New Zealand Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 179: New Zealand Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 180: New Zealand Market Share Analysis (%) By End User, 2022 & 2033
Figure 181: Middle East & Africa Market Value (US$ Million) Analysis, 2018 to 2022
Figure 182: Middle East & Africa Market Value (US$ Million) Forecast, 2023 to 2033
Figure 183: Middle East & Africa Market Value Share, By Test Type (2023 E)
Figure 184: Middle East & Africa Market Value Share, By Sample Type (2023 E)
Figure 185: Middle East & Africa Market Value Share, By End User (2023 E)
Figure 186: Middle East & Africa Market Value Share, By Country (2023 E)
Figure 187: Middle East & Africa Market Attractiveness Analysis by Test Type, 2023 to 2033
Figure 188: Middle East & Africa Market Attractiveness Analysis by Sample Type, 2023 to 2033
Figure 189: Middle East & Africa Market Attractiveness Analysis by End User, 2023 to 2033
Figure 190: Middle East & Africa Market Attractiveness Analysis by Country, 2023 to 2033
Figure 191: GCC Country Market Value Proportion Analysis, 2022
Figure 192: Global Vs GCC Country Growth Comparison, 2022 to 2033
Figure 193: GCC Country Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 194: GCC Country Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 195: GCC Country Market Share Analysis (%) By End User, 2022 & 2033
Figure 196: Türkiye Market Value Proportion Analysis, 2022
Figure 197: Global Vs. Türkiye Growth Comparison, 2022 to 2033
Figure 198: Türkiye Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 199: Türkiye Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 200: Türkiye Market Share Analysis (%) By End User, 2022 & 2033
Figure 201: South Africa Market Value Proportion Analysis, 2022
Figure 202: Global Vs. South Africa Growth Comparison, 2022 to 2033
Figure 203: South Africa Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 204: South Africa Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 205: South Africa Market Share Analysis (%) By End User, 2022 & 2033
Figure 206: North Africa Market Value Proportion Analysis, 2022
Figure 207: Global Vs North Africa Growth Comparison, 2022 to 2033
Figure 208: North Africa Market Share Analysis (%) By Test Type, 2022 & 2033
Figure 209: North Africa Market Share Analysis (%) By Sample Type, 2022 & 2033
Figure 210: North Africa Market Share Analysis (%) By End User, 2022 & 2033






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Antigen Skin Test Market Growth – Trends & Forecast 2025 to 2035
Rapid Antigen Testing Market - Demand, Growth & Forecast 2025 to 2035
Cancer Antigens Market
Chimeric Antigen Receptor (CAR) T-Cell Therapy Market Analysis and Forecast by Type, Application, End User, and Region Through 2035
The Cryptococcal Antigen Lateral Flow Assay Test Market is segmented by Lateral Flow Readers and Kits and Reagents from 2025 to 2035
Mycoplasma Plate Antigens Market Size and Share Forecast Outlook 2025 to 2035
Carcinoembryonic Antigen (CEA) Market
Testing, Inspection & Certification Market Growth – Trends & Forecast 2025 to 2035
5G Testing Market Size and Share Forecast Outlook 2025 to 2035
AB Testing Software Market Size and Share Forecast Outlook 2025 to 2035
5G Testing Equipment Market Analysis - Size, Growth, and Forecast 2025 to 2035
Eye Testing Equipment Market Size and Share Forecast Outlook 2025 to 2035
HSV Testing Market Size and Share Forecast Outlook 2025 to 2035
IoT Testing Equipment Market Size and Share Forecast Outlook 2025 to 2035
HPV Testing and Pap Test Market Size and Share Forecast Outlook 2025 to 2035
GMO Testing Services Market Insights – Food Safety & Regulatory Compliance 2024 to 2034
GMP Testing Services Market
LTE Testing Equipment Market Growth – Trends & Forecast 2019-2027
Drug Testing Systems Market Size and Share Forecast Outlook 2025 to 2035
Sand Testing Equipments Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA