The market for PD-L1 biomarker testing has the very high growth rate due to the increase in immunotherapy drug use for treatment along with cancers such as NSCLC, melanoma, and renal cell carcinoma. F. Hoffmann-La Roche Ltd., Agilent Technologies, Inc., Merck & Co., Inc., Bristol-Myers Squibb, Thermo Fisher Scientific 62.40% in the market designing assays, streamlining, and ensuring that the product is ready for regulators.
The balance of the companies, which includes ECO Medical, MicroPort Scientific Corporation, Canyon Medical Inc. etc. 15.73% market share, better IHC along with molecular diagnostics.
Mid-tier and emerging players are growing with cost-effective high-sensitivity assays for biomarkers and investing in AI-driven tools for the analysis of images to improve accuracy during diagnosis. Further drivers of this market are the increasing pharma-diagnostic partnerships that aim to develop companion diagnostics.
Manufacturers are working towards standardization of assays, regulatory clearances, and automation in pathology workflows to make the market more accessible. The future of PD-L1 testing will be influenced by continued integration of digital pathology and AI-powered diagnostics.
| Attributes | Description |
|---|---|
| Estimated Market Size (2025) | USD 777.2 million |
Explore FMI!
Book a free demo
| Global Market Share | Industry Share (%) |
|---|---|
| Top 3 (F. Hoffmann-La Roche Ltd., Agilent Technologies, Inc., Merck & Co.,) | 48.40% |
| Top 5 (F. Hoffmann-La Roche Ltd., Agilent Technologies, Inc., Merck & Co., Inc., Bristol-Myers Squibb, Thermo Fisher Scientific) | 14.00% |
| Emerging and Regional Players | 37.60% |
Market Concentration Assessment
The PD-L1 biomarker testing market exhibits moderate concentration, with leading players collectively holding approximately 62.40% market share.
The Market Can be classified into four assays which are PD-L1 testing into PD-L1 22C3, PD-L1 28-8, PD-L1 SP142, and PD-L1 263. Assay kits, namely PD-L1 22C3, constitute 42.6% of the total market and have found wide use for determining appropriate candidates for immunotherapy.
It, thus, advances the approach in precision medicine to higher precision. There is an increasing demand for PD-L1 SP142 and PD-L1 263 assays due to their adoption in various types of cancer and personalized therapy approaches. The increasing sophistication of companion diagnostics is now integrating PD-L1 testing with genomic markers, focusing on multi-biomarker assays.
The application segment is headed by NSCLC, holding 48.1% market share, as this cancer has the highest incidence and PD-L1 testing directs treatment decisions for immune checkpoints.
Because PD-L1 expression levels are associated with the response to ICI, testing has become a standard of care in NSCLC. Melanoma and renal cell carcinoma are also important indications with advances in ICI as well as combination therapy. The market is also growing in gastrointestinal tract malignancies and hematological malignancies in which biomarker-guided therapy selection is picking up pace. Clinical trials are rapidly working on the role of PD-L1 in other types of cancers also, thus opening the horizon further for immunotherapy applications.
Hospitals are the leading end-users with a market share of 52.4% because PD-L1 testing has become an integral part of oncology workflow for routine purposes.
Widespread adoption of the test by hospitals is seen because oncologists depend on the rapid and accurate testing of PD-L1 for the optimization of treatment strategies. Advanced molecular pathology techniques and AI-assisted interpretation of biomarkers also make significant contributions from diagnostic laboratories and cancer research institutes.
These institutions have played a key role in fine-tuning test accuracy, developing new methodologies, and integrating digital pathology solutions that streamline biomarker evaluation and enhance reproducibility across different healthcare setting.
Roche Ltd
The company gives more emphasis on automated immunohistochemistry (IHC) platforms. This will make assay performance standardized, ensuring precise and reproducible results. Roche is living up to innovation as the group continues to try to make innovations in the fields of assay automation and efficiency so that it increases the productivity rate of pathologists while giving higher quality output through biomarker quantification. \
To achieve the abovementioned innovations, Roche is heavily investing in AI digital pathology tools aimed at providing telepathology as a way to increase PD-L1 testing throughout the globe. Roche is further enhancing its collaborations in research with oncology centers to validate testing of PD-L1 across several emerging cancer indications that further improve its position within precision medicine and cancer care.
Agilent Technologies
Agilent Technologies, Inc. boasts high-precision PD-L1 assays, of which there are very few around the world that are important to the co-development of companion diagnostics for several immunotherapies. The company works closely with biopharmaceutical companies as it develops testing capabilities into emerging cancer types, fine-tuning methodologies for assessing biomarkers in the process.
Agilent employs the most innovative tissue-staining technologies and innovative digital imaging systems to ensure a high level of consistency and accuracy in scoring of PD-L1. Besides that, Agilent is focused on innovative assay development that will serve to further enhance personalized medicine initiatives, and, by optimizing PD-L1 detection, assist the selection of appropriate targeted therapies. By uniting expertise in diagnostics with an approach to innovation, Agilent contributes greatly to advancing oncology.
Merck & Co
Merck & Co., as MSD outside of the United States and Canada, plays a primary role in innovation around PD-L1 testing for personalized medicine. It focuses intensely on developing better biomarker test technologies that describe better patient outcomes in response to immunotherapies. Diagnostic companies work close to Merck in optimizing assays to ensure it gets the perfect patient population from which to serve the innovative approaches for cancer medication.
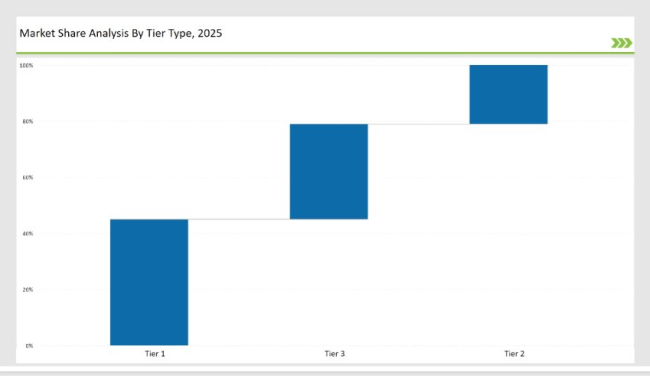
| Tier | Market Share (%) |
|---|---|
| Tier 1 F. Hoffmann-La Roche Ltd., Agilent Technologies, Inc., Merck & Co., Inc., Bristol-Myers Squibb, Thermo Fisher Scientific) | 62.4% |
| Tier 2 (ECO Medical, MicroPort Scientific Corporation, Canyon Medical Inc.and Others.) | 23.7% |
| Tier 3 (Emerging and Regional Players) | 13.90% |
| Company | Unique Initiative |
|---|---|
| Roche | A new initiative is being taken to integrate AI-driven digital pathology and cloud-based image analysis with the testing platforms. This could incorporate standardized scoring algorithms, guaranteeing constant and reproducible outcomes. Its novel approach promises not only to lighten the workload of the pathologists but promises accuracy in the quantitation of the biomarkers. Due to continuous investment in the newest available technologies, Roche is placed among the great leaders of precision oncology's landscape; and thus the test for PD-L1 became reliable and available for healthcare providers globally. |
| Agilent Technologies | Agilent Technologies, Inc. It is highly known for strategic companions in diagnostics for advancing applications of precision oncology. Its company would focus on aiding the biomarker-driven selection of treatment for patients to further strengthen the effectiveness of immunotherapies and stratify better patients through highly precise co-development of assays. |
| Merck & Co., Inc. | The company is proactively collaborating with research and diagnostic organizations to make companion diagnostic assays that may well identify accurately the patients best poised to benefit from its immunotherapy products. It continues investing in further improvement in its knowledge about PD-L1 as a biomarker in many tumor types for much more effective treatment and stratification of the patient population. The research collaborations and programs of Merck help forward the paradigm of biomarker testing and gain access to care in oncology. |
| Bristol-Myers Squibb | .BMS is committed to developing and validating companion diagnostics for the immunotherapy drugs for maximum patient selection. With precision oncology, BMS goes further in its proposition to point out that sophisticated assays help make the right estimation of the biomarker. This is very critical in ascertaining the eligibility of the patients for treatment. BMS continuously endeavors to progress in understanding and application of PD-L1 as a predictive biomarker through much work and collaborations that further enrich its portfolio in targeted cancer therapies |
| Thermo Fisher | In response to the increased demand for greater accuracy in scoring PD-L1, Thermo Fisher Scientific sits at the top of AI-enabled biomarker interpretation. The use of deep learning models and the integration of predictive analytics with the company's extensive digital pathology toolset enhances both the precision and reproducibility of PD-L1 tests. This innovative technology addresses interobserver variability challenges as well as enables streamlined laboratory workflow for large-scale testing. |
Advancements in Multiplex Biomarker Testing: Combination with other biomarkers will add an extra edge toward the patient's selection in receiving immunotherapy, as TMB and MSI marker use coupled with expression analysis of PD-L1 will further determine the comprehensive measure of an occurring immune response likelihood. Progress made in multiplex testing supports improvement in the strategy of precision medicine, guide tailored treatment choices and, thusly, maximize response prediction in the case of immunotherapy.
Standardization of PD-L1 Assay Scoring Methods: Standardization in the scoring methods to interpret PD-L1 tests will make them clinically useful and reproducible across various platforms. Applying the same cut-off values to different assays like 22C3, SP263, 28-8, SP142, along with uniform guidelines for scoring, improves test reliability.
Expansion of Liquid Biopsy-Based PD-L1 Testing: Liquid biopsy-based PD-L1 testing is expected to revolutionize biomarker testing in oncology using non-invasive tests by analyzing circulating tumor DNA. Liquid biopsies are the least minimally invasive alternatives to traditional tissue biopsies; they can be used in the study of real-time dynamics and mechanisms of resistance in PD-L1 expression.
New technologies like next-generation sequencing and digital PCR have taken the enhanced resolution to a much more advanced level, with improved sensitivity and specificity by the development of the further advancement in relation to ctDNA-based PD-L1 testing and also promisingness in the direction of patient stratification and treatment tailoring through the optimal level.
Roche Ltd., Agilent Technologies, Inc., Merck & Co., Inc., Bristol-Myers Squibb, Thermo Fisher Scientific command about 62.4 % share in the global market.
The global PD-L1 Biomarker Testing Market market represents a sale of USD 777.2 million in 2025.
Regional and domestic companies hold nearly 21.87% of the overall market.
PD-L1 testing into PD-L1 22C3, PD-L1 28-8, PD-L1 SP142, and PD-L1 263. Assay kits, namely PD-L1 22C3 Products offering significant growth prospects to market players.
The Market Can be classified into four assays which are PD-L1 testing into PD-L1 22C3, PD-L1 28-8, PD-L1 SP142, and PD-L1 263.
NSCLC, Melanoma, renal cell carcinoma, gastrointestinal tract malignancies, hematological malignancies, cancer
hospitals, diagnostic laboratories and cancer research institutes.
Epidemic Keratoconjunctivitis Treatment Market Overview – Growth, Trends & Forecast 2025 to 2035
Eosinophilia Therapeutics Market Insights – Trends & Forecast 2025 to 2035
Endometrial Ablation Market Analysis - Size, Share & Forecast 2025 to 2035
Endotracheal Tube Market - Growth & Demand Outlook 2025 to 2035
Encephalitis Treatment Market - Growth & Future Trends 2025 to 2035
Edward’s Syndrome Treatment Market – Growth & Future Prospects 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.