The NTRK fusion gene positive advanced solid tumors market is estimated to be valued at USD 145.8 million in 2025 and is projected to reach USD 258.6 million by 2035, registering a CAGR of 5.9 % over the forecast period.
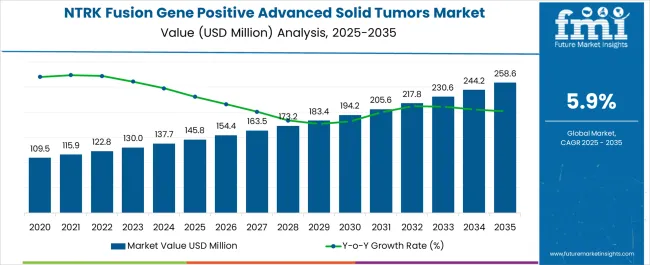
| Metric | Value |
|---|---|
| NTRK Fusion Gene Positive Advanced Solid Tumors Market Estimated Value in (2025 E) | USD 145.8 million |
| NTRK Fusion Gene Positive Advanced Solid Tumors Market Forecast Value in (2035 F) | USD 258.6 million |
| Forecast CAGR (2025 to 2035) | 5.9% |
Growth in this market is supported by the rising importance of precision oncology and increased identification of actionable genomic alterations in rare and aggressive tumors. Advances in genomic sequencing and companion diagnostics have enabled earlier and more reliable detection of NTRK fusions.
Pharmaceutical companies are expanding pipelines of targeted therapies aimed at TRK proteins, which is driving treatment uptake. Regulatory frameworks and accelerated approval pathways also contribute to innovation in this domain.
Hospitals and research centres are increasingly embedding genetic testing into standard oncology workflows, enabling timely treatment interventions and shifting from conventional chemotherapy toward highly targeted approaches.
The market is segmented by Indication and End User and region. By Indication, the market is divided into NTRK 1 Fusion Tumors, NTRK 2 Fusion Tumors, and NTRK 3 Fusion Tumors. In terms of End User, the market is classified into Hospitals, Specialty Clinics, Cancer Centers, and Cancer Research Institutes. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.

The NTRK1 fusion-tumors segment is projected to represent 47.6 % of total market revenue by 2025, making it the leading indication. This is attributed to the relatively higher prevalence of NTRK1 gene fusions compared to other subtypes, combined with advances in next-generation sequencing facilitating earlier detection. Clinical evidence of durable responses and tumor-agnostic benefits of TRK inhibitors in patients with NTRK1 fusions has bolstered physician confidence. Ongoing trials and expansion of companion diagnostic testing are improving patient identification and access to therapy.
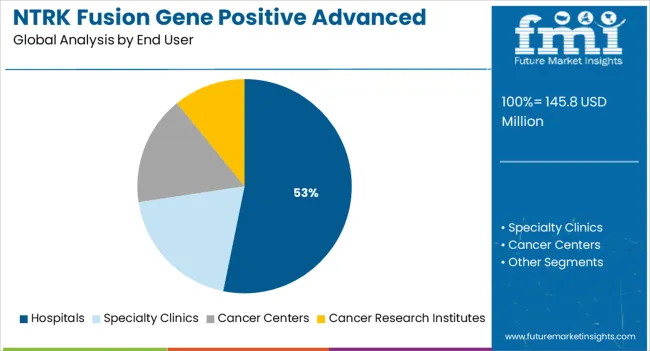
The hospitals segment is expected to account for 53.2 % of overall market revenue by 2025, establishing it as the largest end-user segment. This predominance is because hospitals house advanced oncology infrastructure, have access to genomic testing, and maintain multidisciplinary care teams. They are also the primary venues for clinical-trial enrolment, allowing early uptake of novel TRK inhibitors and combination therapies. Supportive reimbursement schemes and integrated care pathways further accelerate the uptake of NTRK-targeted treatments in hospitals. With precision oncology and comprehensive patient care becoming standard, hospitals remain the central delivery point for advanced therapeutic solutions in this market.
Advancements in precision medicine are among the main drivers of this market. Precision oncology focuses on tailoring treatment to a patient’s genetic profile, lifestyle, and medical history, enabling more effective interventions for patients with NTRK fusion-positive solid tumours. More effective targeted treatments and personalised plans drive demand for genetic testing and new therapies.
Better understanding of cancer genetics, especially NTRK fusions across multiple tumour types, has led to the development of innovative therapies targeting these alterations. As a result, patients with NTRK-fusion-positive advanced solid tumours are now able to access more efficient treatments tailored to their condition.
The increased demand for genetic testing to identify specific mutations further drives market growth. For example, in January 2025, KEYTRUDA (pembrolizumab) by Merck & Co., Inc. was approved by the U.S. Food and Drug Administration (FDA) for adult patients with stage IB, II or IIIA non-small cell lung cancer (NSCLC) after surgery and platinum-based chemotherapy; this underscores how precision treatments are shifting cancer-care paradigms.
The North American market for NTRK fusion gene positive advanced solid tumors has seen significant growth in recent years. Leading pharmaceutical companies have made substantial investments in research and development, resulting in the approval of several drugs that target NTRK fusion gene positive advanced solid tumors.
As a result, North America is poised to continue its leadership role in the development of the NTRK fusion gene positive advanced solid tumors market in the coming years.
The most lucrative end user for the NTRK fusion gene positive advanced solid tumors market is the healthcare sector, specifically hospitals. With a high prevalence of advanced solid tumours and a growing focus on precision medicine, hospitals are at the forefront of the use of NTRK fusion gene positive advanced solid tumors treatments.
A patient with tumors who has mutated genes NTRK is called NTRK fusion gene positive tumors. Although NTRK fusions are rare and account to nearly 1% of solid tumors, the exact frequency is quite unclear. DNA Next Generation Sequencing (NSG) technology is a precise method to detect NTRK fusion genes. It has high specificity and sensitivity.
It can detects gene partners that may have high clinical implications in treatment. Currently Commercialized techniques such as DNA NGS panels, Foundation One may not detect NTRK fusions.
NTRK fusions has higher prevalence rate as they are found in most of solid cancer types. This is expected to drives NTRK fusion gene positive advanced solid tumors market. NTRK is a unique gene which has implications in many types of cancers and the development of latest drugs is expected to drive NTRK fusion gene positive advanced solid tumors market.
However, Poor healthcare infrastructure and high costs associated with genetic screening of NTRK fusion genes may hamper growth of NTRK fusion gene positive advanced solid tumors market in the future.
The global NTRK fusion gene positive advanced solid tumours market is expected to show steady growth over the forecast period due to technological advances, improved screening, and new drug launches. Early detection of NTRK fusion genes in patients contributes to market potential. The high potential is also driven by novel therapeutics in development for various tumour types with NTRK fusions. For instance, the FDA has approved TRK inhibitors such as Larotrectinib (VITRAKVI®) and Entrectinib for treatment of solid tumours with NTRK gene fusions.
The global NTRK fusion gene positive advanced solid tumors market is expected to be dominated by North America owing to rising cancer clinical research and development activities. Latin America market is emerging in NTRK fusion gene positive advanced solid tumors market due to increased spending on healthcare infrastructure.
Europe market is expected to be the second most growing region in the global NTRK fusion gene positive advanced solid tumors market owing to early diagnosis and treatment adoption.
NTRK fusion gene positive advanced solid tumors market in East Asia and South Asia is expected to show significant growth due to improving healthcare infrastructure.
Middle East and Africa market is expected to be least lucrative region in the NTRK fusion gene positive advanced solid tumors market due to poor healthcare facilities and less disease screening facilities.
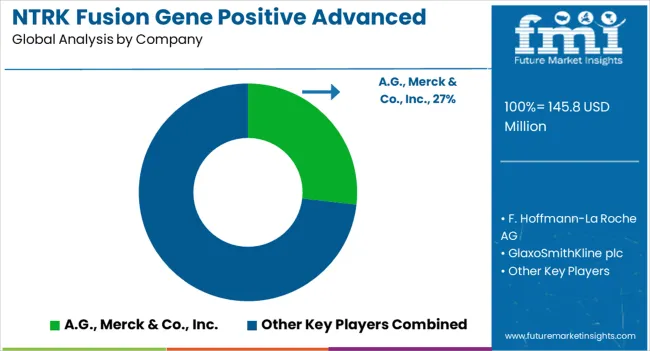
Examples of some of the market participants in NTRK fusion gene positive advanced solid tumors market identified across the value chain includes Bayer A.G., Merck & Co., Inc., F. Hoffmann-La Roche AG, Glaxosmithkline plc, Pfizer Inc., Plexxikon, Daiichi Sankyo Company Limited, Deciphera Pharmaceuticals, Inc., Eli Lilly and company, Exelixis, Inc., Cephalon, Inc., and others.
The research report on NTRK fusion gene positive advanced solid tumors market presents a comprehensive assessment of the market and contains thoughtful insights, facts, historical data, and, statistically supported and industry-validated market data. It also contains projections using a suitable set of assumptions and methodologies.
The global NTRK fusion gene positive advanced solid tumors market is estimated to be valued at USD 145.8 million in 2025.
The market size for the NTRK fusion gene positive advanced solid tumors market is projected to reach USD 258.6 million by 2035.
The NTRK fusion gene positive advanced solid tumors market is expected to grow at a 5.9% CAGR between 2025 and 2035.
The key product types in NTRK fusion gene positive advanced solid tumors market are NTRK 1 fusion tumors, NTRK 2 fusion tumors and NTRK 3 fusion tumors.
In terms of end user, hospitals segment to command 53.2% share in the NTRK fusion gene positive advanced solid tumors market in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Fusion Beverage Market Trends - Size, Demand & Forecast 2025 to 2035
Fusion Bag Market Analysis Size and Share Forecast Outlook 2025 to 2035
Fusion Beverages in Japan Market Analysis – Size, Share & Growth 2025-2035
Infusion Pumps Market Size and Share Forecast Outlook 2025 to 2035
Infusion fluid holder Market Size and Share Forecast Outlook 2025 to 2035
Infusion Pharmacy Management Market
Infusion Chair Market
Infusion Rate Monitoring Market
Diffusion Dialysis Membrane Market Size and Share Forecast Outlook 2025 to 2035
Perfusion Imaging Market Growth – Industry Trends & Forecast 2024-2034
Perfusion Tubing Systems Market
Non-Fusion Spinal Devices Market Growth - Trends & Forecast 2025 to 2035
IV Infusion Bottle Seals & Caps Market Size and Share Forecast Outlook 2025 to 2035
NRG1 Fusion-Targeted Therapy Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
IV Infusion Gravity Bags Market Size and Share Forecast Outlook 2025 to 2035
Transfusion Dependent Thalassaemia Management Market - Trends & Future Outlook 2025 to 2035
Examining Market Share Trends in IV Infusion Bottle Seals & Caps
Transfusion Bottles Market
Korea Fusion Beverage Market Analysis by Beverage Type, Ingredient Profile, Distribution Channels, and Country Through 2035
Sensor Fusion Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA