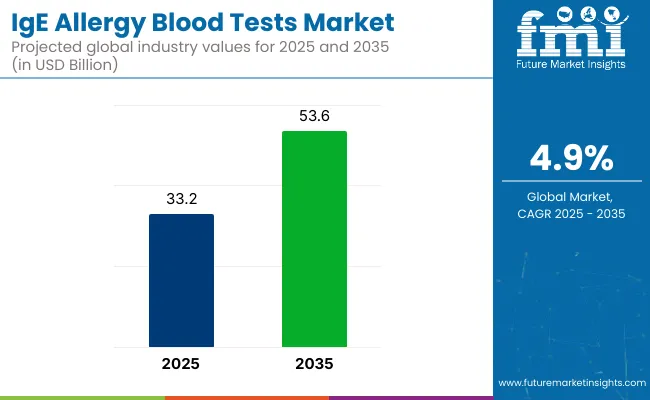
The IgEallergy blood tests market is valued at USD 33.2 billion in 2025 and is slated to reach USD 53.6 billion by 2035, at a CAGR of 4.9%. Its growth is driven by the rising prevalence of allergic diseases, increasing awareness towards early diagnosis, and technological advancements in immunoassay testing platforms.
| Metric | Value |
|---|---|
| Industry Size (2025) | USD 33.2 Billion |
| Industry Value (2035) | USD 53.6 Billion |
| CAGR (2025 to 2035) | 4.9% |
Market expansion is also supported by the shift towards personalized allergy diagnostics and AI-powered allergy prediction models for precision care..The market holds an estimated 100% share within the allergy diagnostics segment, underscoring its central role in this field. Within the broader in vitro diagnostic (IVD) market, it commands approximately 1-2%, reflecting its niche position.
It contributes around 10-15% to the immunoassay market, where it plays a significant role in allergy testing. In the clinical diagnostics market, its share is approximately 5-10%, emphasizing its specialized function. Within the personalized medicine market, it accounts for roughly 2-4%, supporting tailored allergy treatments.
The market has witnessed significant innovations aimed at improving diagnostic accuracy, efficiency, and accessibility. Advancements such as nano-ELISA technology, AI-driven interpretation software, and microfluidic biosensor platforms are enhancing assay sensitivity, reducing turnaround times, and enabling high-throughput multiplex allergen testing in clinical laboratories.
These technological developments are being integrated with telehealth services and home-based testing kits to increase accessibility, particularly for patients in remote or underserved regions, thereby expanding market penetration and enhancing early allergy detection.
The USA is expected to be the fastest-growing market for IgE allergy blood tests, with a projected CAGR of 5.2% from 2025 to 2035. The ELISA testing kits segment will dominate the product type category, accounting for over 65% of the market share in 2025. Aero allergies will lead the application segment, capturing 70% of the market share in 2025. France is forecasted to grow at a CAGR of 4.9%, while Germany is projected to grow at a CAGR of 4.8%.
The market is segmented into product type, application, end user, and region. By product type, the market is divided into assays and kits, ELISA testing kits, fluorescent enzyme immunoassays (FEIA) testing kits,chemiluminescent immunoassays (CLIA), reagents, consumables, and analyzers. Based on application, the market is categorized into aero allergies, indoor allergies, outdoor allergies, food allergies, venoms, medicine allergies, and latex/metal allergies.
In terms of end user, the market is segmented into diagnostic laboratories, specialty clinics, hospitals, and research & academic centers. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, East Asia, South Asia Pacific, and Middle East and Africa.
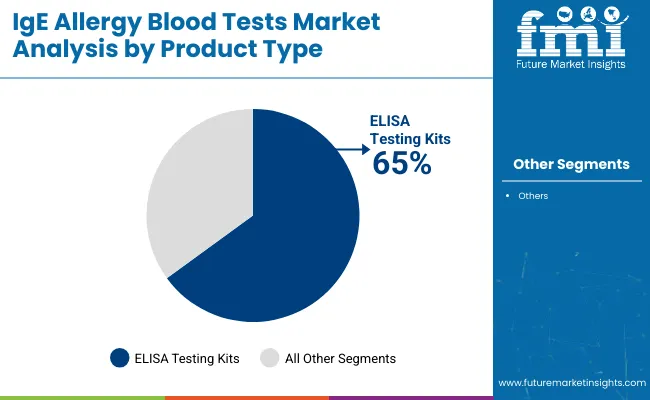
ELISA testing kits are projected to lead the product type segment, accounting for over 65% of the global market share by 2025. These kits are widely used for their specificity, automation compatibility, and quantitative results, making them suitable for diagnostic laboratories.
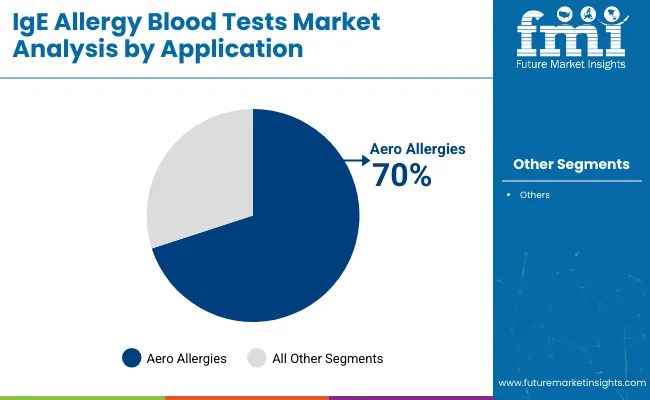
Aero allergies are expected to lead the application segment, capturing approximately 70% market share in 2025. Rising exposure to airborne allergens such as pollen, dust mites, and mold spores has increased demand for high-specificity aeroallergen blood tests.
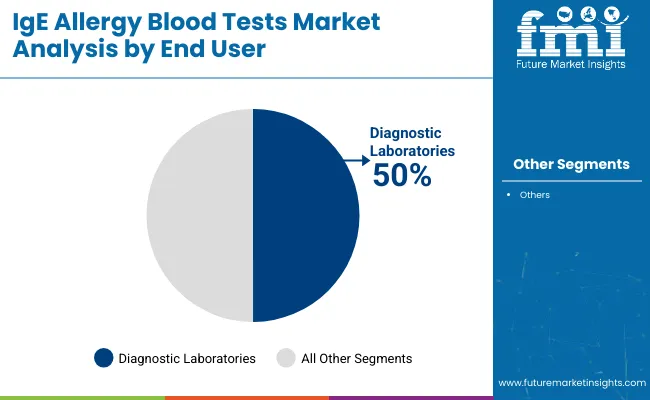
Diagnostic laboratories are projected to lead the end user segment, accounting for over 50% of the global market share by 2025. These facilities have remained primary providers of allergy diagnostics due to their advanced infrastructure, high-throughput testing capabilities, and integration of automated immunoassay platforms.
The IgE allergy blood tests market has been experiencing steady growth, driven by the rising prevalence of allergic diseases, technological advancements in immunoassay platforms, and increased awareness regarding early diagnosis and personalized allergy management.
Recent Trends in the IgE Allergy Blood Tests Market
Challenges in the IgE Allergy Blood Tests Market
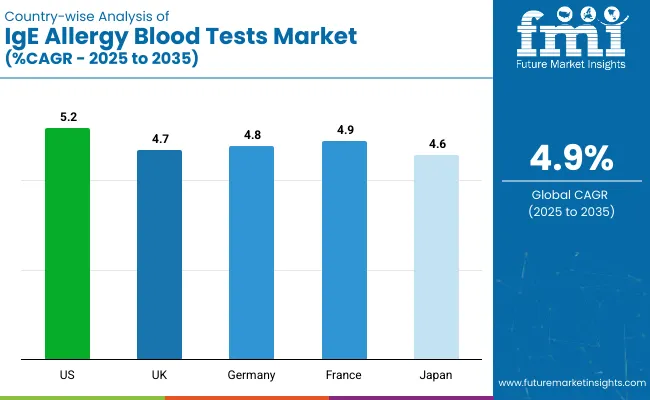
The USA leads with a CAGR of 5.2%, driven by precision diagnostics and telehealth expansion. France follows with 4.9% CAGR, supported by molecular diagnostics and eco-friendly reagents adoption. Germany’s CAGR stands at 4.8%, backed by multiplex testing panels and EU-standardized protocols.
The UK market grows at 4.7% CAGR, with food allergy diagnostics and point-of-care testing as key drivers. Japan shows a 4.6% CAGR, supported by molecular-based assays and home-based solutions. Overall, the USA shows the highest growth, while Japan records the lowest CAGR among these leading countries in IgE allergy blood testing.
The report covers in-depth analysis of 40+ countries, with five top-performing OECD countries highlighted below.
for IgE allergy blood tests demand in the USA is projected to grow at a CAGR of 5.2% from 2025 to 2035. Growth has been supported by rising prevalence of allergies, increasing adoption of precision diagnostics, and advancements in AI-powered testing solutions.
The UK IgE allergy blood tests revenue is forecast to expand at a CAGR of 4.7% over the forecast period. Demand has been driven by high food allergy prevalence, government initiatives for early detection, and adoption of point-of-care testing solutions.
IgE allergy blood tests demand in Germany is projected to grow at a CAGR of 4.8% between 2025 and 2035. The nation’s strong healthcare infrastructure and focus on allergen standardization are boosting demand for advanced testing solutions.
The French IgE allergy blood tests market is expected to grow at a CAGR of 4.9% from 2025 to 2035. Growth has been supported by rising food and respiratory allergies, increasing adoption of molecular diagnostics, and AI-based allergy analysis.
The sales of IgE allergy blood tests in Japan are forecast to grow at a CAGR of 4.6% through 2035. Growth has been driven by rising allergy prevalence, advanced biotechnology capabilities, and demand for high-precision diagnostic solutions.
The IgE allergy blood tests market is moderately consolidated, with global leaders and regional firms driving competitive dynamics through high-sensitivity immunoassays, AI-integrated diagnostics, and automated testing platforms.
Tier-one firms, such as Thermo Fisher Scientific, Siemens Healthineers, Danaher Corporation (Beckman Coulter), Omega Diagnostics Group PLC, and HYCOR Biomedical, have been focusing on technological innovation, high-throughput capabilities, and expanded allergen panel offerings for enhanced diagnostic accuracy and patient care.
Recent IgE Allergy Blood Tests Industry News
| Report Attributes | Details |
|---|---|
| Current Total Market Size (2025) | USD 33.2 billion |
| Projected Market Size (2035) | USD 53.6 billion |
| CAGR (2025 to 2035) | 4.9% |
| Base Year for Estimation | 2024 |
| Historical Period | 2020 to 2024 |
| Projections Period | 2025 to 2035 |
| Report Parameters | Revenue in USD billion / Volume in units |
| By Product Type | Assays and Kits, ELISA Testing Kits, Fluorescent Enzyme Immunoassays (FEIA) Testing Kits, Chemiluminescent Immunoassays (CLIA), Reagents, Consumables, and Analyzers |
| By Application | Aero Allergies, Indoor Allergies, Outdoor Allergies, Food Allergies, Venoms, Medicine Allergies, and Latex/Metal Allergies |
| By End User | Diagnostic Laboratories, Specialty Clinics, Hospitals, and Research & Academic Centers |
| Regions Covered | North America, Latin America, Western Europe, Eastern Europe, East Asia, South Asia Pacific, Middle East and Africa |
| Countries Covered | United States, Canada, United Kingdom, Germany, France, China, Japan, South Korea, Brazil, Australia |
| Key Players | Thermo Fisher Scientific, Siemens Healthineers, Danaher Corporation (Beckman Coulter), Omega Diagnostics Group PLC, HYCOR Biomedical, Abcam plc, PerkinElmer Inc., R- Biopharm AG, Eurofins Scientific, BioMérieux SA |
| Additional Attributes | Market share analysis by product type and application, country-wise CAGR analysis, and brand positioning insights |
The market is expected to reach USD 53.6 billion by 2035.
The global market is projected to grow at a CAGR of 4.9% during this period.
ELISA testing kits are expected to lead with over 65% market share in 2025.
Aero allergies are expected to hold approximately 70% of the market share in 2025.
The USA is anticipated to be the fastest-growing market with a CAGR of 5.2% from 2025 to 2035.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
SiGe:C HBT Market Size and Share Forecast Outlook 2025 to 2035
Digestion Resistant Maltodextrin Industry Analysis in Korea Size and Share Forecast Outlook 2025 to 2035
Digestive Enzyme Market Size and Share Forecast Outlook 2025 to 2035
Digestion Aids Market Size and Share Forecast Outlook 2025 to 2035
Digestion Resistant Maltodextrin Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Digestive Enzyme Supplements Market Size, Growth, and Forecast for 2025 to 2035
GigE Camera Market Size and Share Forecast Outlook 2025 to 2035
Tiger Nut Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Digestive Health Supplements Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Digestion-Resistant Maltodextrin Market in Japan - Growth & Demand from 2025 to 2035
Digestion-Resistant Maltodextrin Industry Analysis in Western Europe - Growth & Market Insights 2025 to 2035
Digestive Health Drinks Market Analysis by Ingredient Type, Sales Channel, Region Through 2025 to 2035
Pigeon Pea Market Insights – Growth & Forecast 2025-2035
Apigenin Market Size and Share Forecast Outlook 2025 to 2035
Geiger Counters Market Analysis by Application, Type, and Region: Forecast for 2025 to 2035
Indigenous Tourism Market Size and Share Forecast Outlook 2025 to 2035
Antigen Skin Test Market Growth – Trends & Forecast 2025 to 2035
Refrigerated Display Case Market Size and Share Forecast Outlook 2025 to 2035
Refrigeration and Air Conditioning Compressors Market Size and Share Forecast Outlook 2025 to 2035
Refrigeration Compressor Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA