Germany preclinical medical device testing services market is expected to reach USD 92.3 million in 2025. The market is projected to grow at a CAGR of 4.1% and reach a total value of USD 138.3 million by 2035.
| Attributes | Values |
|---|---|
| Estimated Industry Size 2025 | USD 92.3 million |
| Projected Value 2035 | USD 138.3 million |
| Value-based CAGR from 2025 to 2035 | 4.1% |
The Germany preclinical medical device testing services market is expected to witness significant growth. The country has well established healthcare infrastructure and is also a leader in innovation and manufacturing of medical devices, which makes it one of the prominent country to hold highest share in preclinical testing services.
In addition, Germany invests heavily in cutting-edge technologies which has inclusion of simulation and imaging. This technologies requires enhanced testing efficiency and accuracy for approval of their products in the market. Therefore, increase in adoption of sustainable practices along with the growing focus of manufacturers on introducing patient-centric solutions, anticipates the growth of the market. Moreover, continuously evolving well-established ecosystem for clinical trials, also contributes significantly to growth of the market.
Explore FMI!
Book a free demo
The table below offers a detailed comparative assessment of the changes in the compound annual growth rate (CAGR) over six months for the base year (2024) and the current year (2025) specifically for the Germany preclinical medical device testing services market.
This semi-annual analysis highlights demonstrates a changes in market dynamics. The H1, which is the first half of the year shows the January to June, while the second half, H2, spans July to December.
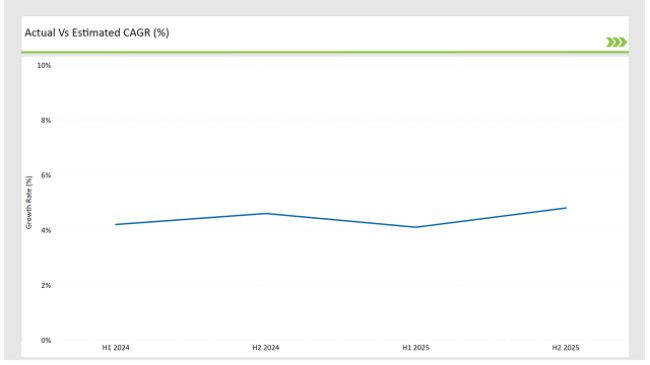
H1 signifies period from January to June, H2 Signifies period from July to December
Preclinical medical device testing services is projected to grow at a CAGR of 4.2% during H1 2024 and further surge to an increment of 4.6% in the latter half of 2024. In 2024, the rate is projected to slightly lower down to 4.1% in H1 and increase up to 4.8% in H2. The market also witnessed decline of 33 basis points from the first half of 2024 to the first half of 2025 and an increase of 28 basis points in the second half of 2025 over the second half of 2024.
The foregoing figures describe the dynamics or changing nature in the Germany preclinical medical device testing services market impacted by factors, such as altered regulatory changes or innovations in several services provided by the service providers.
This semi-annual breakdown is critical for businesses planning their strategies to capitalize on the anticipated growth and navigate the complexities of the market.
| Date | Development/M&A Activity & Details |
|---|---|
| 2025 | Growing Focus on Medical Device Innovation: iuvo BioScience is investing towards innovation in medical devices anticipate the growth of the market in Germany |
| 2024 | Development of well-established Clinical trial Ecosystem: Porsolt has Strong Clinical trial infrastructure in Germany encourage manufacturers to develop new medical device. |
| 2024 | Advancements in Healthcare Infrastructure: Gradient LLC is responsible for the growing need of developing and maintaining strong healthcare infrastructure anticipates market growth in Germany |
Growing Export Market anticipates the Growth of Preclinical Medical Device Testing Services in the Germany
Germany is considered one of the leading countries in the global medical device market, which drives the preclinical medical device testing services market to a great extent. German manufacturers export medical devices to majority of the countries. For this they follow strict international regulatory standards set by the FDA, EMA, and other global agencies.
It is this demand for compliance globally that drives the need for extensive preclinical testing, which ensures that devices meet multiple market safety, efficacy, and performance requirements. Inevitably, it also makes preclinical testing services pivotal in assisting manufacturers through such complex regulatory systems to ensure approval of their devices for use across different regions.
This international focus not only raises demand for testing services within Germany but also secures its status as a leader in the world for the development and testing of medical devices.
Increase in Availability of Advanced Testing Facilities Anticipates its Market Growth in Germany
Germany's access to superior levels of testing facilities. The country has presence of established laboratories and research centers which enable a manufacturer to conduct detailed and accurate preclinical studies of their devices. These factors ensure the fulfillment of safety and efficacy requirements of the products that are manufactured in Germany at an international level.
This is a situation whereby German companies, through having at their disposal such infrastructure, will easily conduct extensive testing and comply with various regulatory requirements such as those of the FDA or EMA. Therein, therefore, manufacturers streamline product development time to market while simultaneously increasing quality and compliance with given requirements. As already indicated earlier, this further makes Germany dominant as the global market leader within the medical device manufacturing sector.
% share of Individual categories by Service Type and End User in 2025
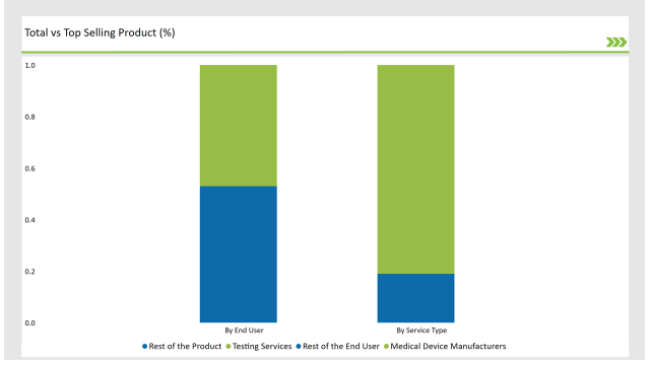
The Critical Factor of Ensuring the Safety and Efficiency in Medical Device Aid Testing Services to dominate the Market
Testing services lead the market because they play a very crucial role in ensuring medical devices are safe and effective, and their manufacture also complies with regulatory affairs. Medical devices have to undergo preclinical testing, which involves various risks, design flaws, and performance problems associated with the device.
These services ensure that devices meet the necessary standards for regulatory approval from bodies like the FDA and EMA. The increasing sophistication of modern medical devices, especially those involving advanced materials, AI, or digital health features, makes specialized testing critical to properly assess safety and functionality.
Moreover, testing services reduce overall costs and development timelines by identifying issues early in the process, preventing costly failures later on. With the advancement of medical technology, the demand for expert testing services has also increased, making them truly incomparable in the market.
Comprehensive Testing Required by Medical Device aid them to hold Dominant Position
Leading players in the preclinical medical device testing services market include medical device manufacturers themselves, since they remain the major designers, developers, and introducers of such devices to the market. Preclinical testing services are mandatory to be performed by these manufacturers before their devices are used in clinical trials or released to the public.
In this process, preclinical testing services become indispensable since they help identify potential risks, flaws, and compliance issues at an early development stage, thus minimizing costly revisions later.
Apart from that, manufacturing companies closely interact with service providers to ensure that the testing methods applied are precisely suitable for their device's specific features and requirements. Such collaboration enables the smoothing of the development process, acceleration of time-to-market, and makes the final product fully meet the requirements of the corresponding regulatory bodies.
Since this is the core part of the product development lifecycle, medical device manufacturers remain the dominant force driving demand in the preclinical testing services market.
The Germany preclinical medical device testing services market is moderately fragmented, with a mix of multinational corporations and regional players contributing to a dynamic competitive environment. Companies like Laboratory Corporation of America Holdings, Charles River Laboratories, WUXI APPTEC and Sotera Health dominate the market by leveraging advanced technologies for streamlining their production process.
2025 Market share of US Preclinical Medical Device Testing Services
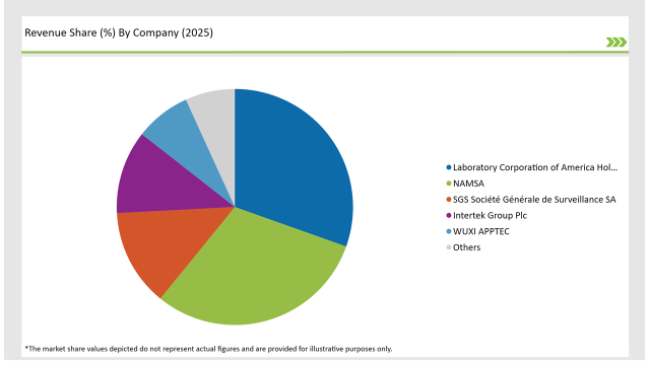
The competitive landscape of the Germany preclinical medical device testing services market features a blend of major multinational corporations and innovative regional companies.
By 2025, the Germany preclinical medical device testing services market is expected to grow at a CAGR of 4.1%.
By 2035, the sales value of the Germany preclinical medical device testing services industry is expected to reach USD 138.3 million.
Key factors that are attributing to the growth of the Germany preclinical medical device testing services market include growing export market and support extended by government and healthcare organizations for development of innovative medical device products.
Prominent players in the Germany preclinical medical device testing services manufacturing include Laboratory Corporation of America® Holdings, NAMSA, SGS Société Générale de Surveillance SA., Intertek Group Plc, WUXI APPTEC, TÜV SÜD, Sotera Health, Eurofins Scientific, iuvo BioScience, llc, RQM+, Pace Analytical Services LLC, Pharmaron, Bioneeds India Pvt. Ltd., Porsolt, Gradient LLC and Goupe Icare.
The industry includes Testing Services (Biocompatibility Testing, Microbiological & Sterility Testing, Analytical chemistry {Material Characterization, Extractables and leachables, Storage and stability testing and Polymer Investigation}, Toxicology Testing { Cytotoxicity, Genotoxicity and Other Toxicology Testing}, Functional Testing, Electromagnetic Compatibility (EMC) Testing, Implantation Studies, Biological Safety Evaluation, Package Validation, Reusability Testing, Pyrogen Testing and Others, and Consulting Services (Device Designing/Engineering and Regulatory affairs Consulting).
In terms of device category, the industry is divided into Orthopedics, Cardiovascular, Respiratory, Diabetes, Dental, Neurology, Oncology, Ocular, Bariatrics, Wound Healing, General Health (Wearables), In Vitro Diagnostics, General Surgery, Drug Device Combination and Other Device Category.
The industry is divided into Class I, Class II and Class III.
The industry is classified by end user as medical device manufacturers, pharmaceutical and biotech companies, device design and engineering firms and academic and research institutions
GMG Management Market - Growth & Treatment Advances 2025 to 2035
Home Infusion Therapy Devices Market - Growth & Forecast 2025 to 2035
Human Combinatorial Antibody Libraries (HuCAL) Market - Trends & Forecast 2025 to 2035
Home Healthcare Market Growth - Trends, Innovations & Forecast 2025 to 2035
Dental 3D Printing Material Market Trends, Growth & Forecast by Material, Product, and Region through 2035
Catheter Market Insights by Product, Indication, End-user, and Region 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.