The GCC structured product label (SPL) management market is projected to reach a value of USD 3,445.3 million in 2025 and will grow at a CAGR of 13.8%, reaching USD 12,495.2 million by 2035.
| Attributes | Values |
|---|---|
| Estimated GCC Industry Size 2025 | USD 3,445.3 million |
| Projected GCC Industry Size 2035 | USD 12,495.2 million |
| Value-based CAGR from 2025 to 2035 | 13.8% |
Growth of the market is propelled by regulatory requirements, increasing production spending, as well as digital transformation initiatives in the GCC download the report summary to find out more. The GCC region pharmaceutical, biotechnology and medical device companies are embracing structured labeling solutions leading to regulatory compliance of operations.
Cloud-based SPL solutions are becoming popular due to the demand for remote accessibility, cost-effectiveness, and scalability. AI-based Compliance Management Systems and blockchain-empowered security mechanisms are some of the advanced technologies that are reorienting the SPL management domain in the GCC industry. Market growth is further propelled by government initiatives and collaboration between regulatory bodies and industry stakeholders.
Exclusive Offer: 30% Off on Regional Reports
Get a free sample report and customize your regions for a 30% discount on your regional report!
The table below provides a semi-annual comparison of the CAGR, showcasing the market’s growth trajectory.
| Particular | Value CAGR |
|---|---|
| H1, 2024 | 13.4% |
| H2, 2024 | 13.6% |
| H1, 2025 | 13.9% |
| H2, 2025 | 14.2% |
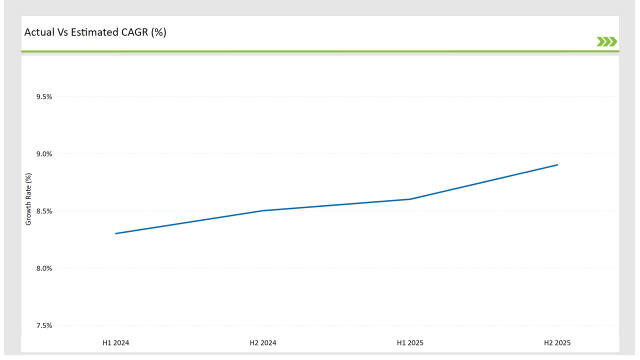
H1 signifies January to June, while July to December analysis is signified through H2.
The adoption of cloud-based labelling platforms, AI-driven automation and the compliance tracking systems helps to accelerate in the GCC region. The market CAGR is expected to increase from 13.4% in H1 2024 to 14.2% by H2 2025, indicating strong market momentum
| Date | Development/M&A Activity & Details |
|---|---|
| Jan-2025 | Saudi Arabia’s SFDA introduces new regulatory requirements for structured labeling in pharmaceuticals. |
| Oct-2024 | UAE’s Ministry of Health partners with global tech firms to develop AI-driven SPL management solutions. |
| Mar-2024 | Qatar launches a digital regulatory framework for medical device labeling. |
| Sep-2024 | Bahrain mandates electronic structured labeling for all imported pharmaceuticals. |
| Dec-2023 | Kuwait integrates blockchain into structured labeling processes for improved data security. |
These developments underscore regulatory advancements, digital transformation, and AI-driven compliance monitoring as key trends defining the market. Increasing investments in digital healthcare solutions and strategic collaborations with global SPL software providers are expected to drive further growth.
AI-Powered Compliance and Automation
The growing complexity of regulatory frameworks for pharmaceuticals and medical devices in the GCC is driving the adoption of AI-powered Structured Product Labelling (SPL) solutions. These intelligent automation solutions reduce human error, improve the speed of document validation, and quickly adapt to real-time, dynamic regulations.
Regulatory workflows are time-consuming, yet SPL platforms powered by artificial intelligence automate updates, flag non-compliant content, and integrate with regulatory databases to ensure that compliance can be validated instantly by the platform. In Saudi Arabia, UAE, and Qatar, domestic pharmaceutical and biotechnology firms are expanding operations and managing extensive regulatory requirements with AI-based compliance tools.
AI-driven SPL solutions further improve submission processes by using ML algorithms to identify discrepancies and suggest corrections. Artificial intelligence integrated with natural language processing (NLP), allows for automated drug label classification for seamless regulatory reporting. AI-powered regulatory tracking also helps companies stay in line with evolving GCC health authority guidelines, minimizing delays and potential fines.
Cloud-Based SPL Solutions Gain Traction
Cloud-based SPL solutions are poised to dominate the GCC pharmaceutical and biotechnology sectors, becoming a mainstream choice due to factors such as cost efficiency, real-time collaboration, and regulatory alignment. Cloud-based SPL platforms allow for more centralized data management, making regulatory document access seamless across various stakeholders.
Saudi Arabia, UAE, and Bahrain-based pharmaceutical companies are increasingly moving towards Software-as-a-Service (SaaS) models to automate compliance tracking and minimize operational costs for multi-market regulatory submissions.
Given the dynamic nature of regulations, they offer real-time alignment to geographically diverse regulatory landscapes, instantly updating labeling specifications through automation. Additionally, GCC regulatory authorities promote cloud adoption to increase transparency, accelerate approval processes, and improve pharmacovigilance reporting.
Rising Demand for Biologics and Specialty Pharmaceuticals
The GCC pharmaceutical market is experiencing growth of biologics, gene therapies, and specialty pharmaceuticals, resulting in a demand for structured product labelling (SPL) solutions. Such high-value therapeutics need robust regulatory documentation, elaborate labelling validation and real-time compliance tracking.
Saudi Arabia, UAE and Qatar are positioning themselves as hubs of biotechnology and pharmaceutical innovation, and this creates the demand for automated SPL solutions designed specifically for biologics. AI-empowered SPL systems guarantee accurate archival of molecular compositions, dosage instructions and safety warnings, significantly reducing regulatory risks Furthermore, the automated SPL platforms assist in integrating the approval process with global health authorities' databases, thus accelerating the submissions for specialized drugs.
With cell and gene therapy treatments on the rise, there is even more pressure on the need for adaptive labelling solutions that can accommodate individualised treatment data.
Blockchain for Enhanced Label Security
The impact of blockchain technology is revolutionizing SPL management in the GCC pharmaceutical and biotechnology industry. By documenting every step in an SPL system via blockchain, you have a permanent record that cannot be tampered with-drastically lowering the risk of counterfeit drugs hitting the market or critical information on labels being altered.
Pharmaceutical companies in Saudi Arabia, UAE, and Oman can effectively use blockchain technology to store and distribute regulatory data securely using decentralized digital ledgers, thus reducing the risk of compliance failure from label changes. The automated identification and sharing of supply data across stakeholders enabled by blockchain coupled with SPL also provides streamlined audit trails of verified labelling data for regulators, improving compliance efficiency.
The use of smart contracts can help automate the validation and expiration tracking process for the generated labels, ensuring that the relevant up-to-date and compliant labelling information in use through the entire supply chain.
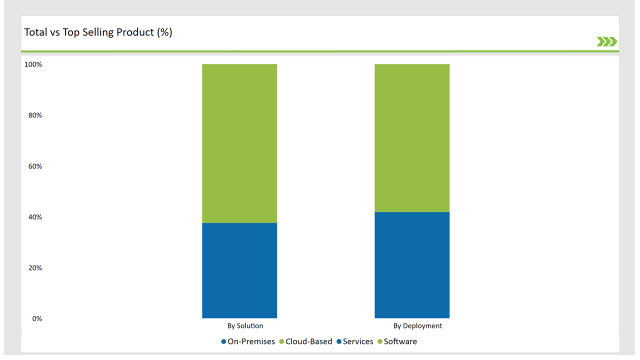
| Solution | Market Share (2025) |
|---|---|
| Software | 62.5% |
| Services | 37.5% |
Software solutions account for 62.5% of the market in 2025, reflecting the growing demand for automated compliance tracking, AI-based validation, and cloud-based SPL management platforms. The services sector plays a crucial role in implementation, regulatory consulting, and system integration.
| Deployment | Market Share (2025) |
|---|---|
| Cloud-Based | 58.2% |
| On-Premises | 41.8% |
Cloud-based SPL solutions are dominating the market with 58.2% share in 2025, and help for reflecting the region’s preference for scalable, cost-effective digital solutions. The on-premises solutions remain is also very essential for organizations requiring strict data control and regulatory compliance.
Check Free Sample Report & Save 40%!
Select your niche segments and personalize your insights for smart savings. Cut costs now!
| Countries | CAGR |
|---|---|
| Saudi Arabia | 16.2% |
| UAE | 14.8% |
| Rest of GCC Countries | 11.5% |
The factors that drive growth in Saudi Arabia's structured product label management market include the country's Vision 2030, with an emphasis on digital transformation in pharmaceuticals and consumer goods. The Saudi Food and Drug Authority enforces strict regulations in labeling, hence pushing companies towards automated labeling solutions.
Increased foreign investment in the manufacturing and logistics sectors accelerates this further. E-commerce expansion also requires accurate, compliant labeling. Industry players focus on integrating AI and blockchain for traceability, enhancing compliance and efficiency. Growing pharmaceutical exports require structured label management to meet international standards, thereby driving the market in key industries.
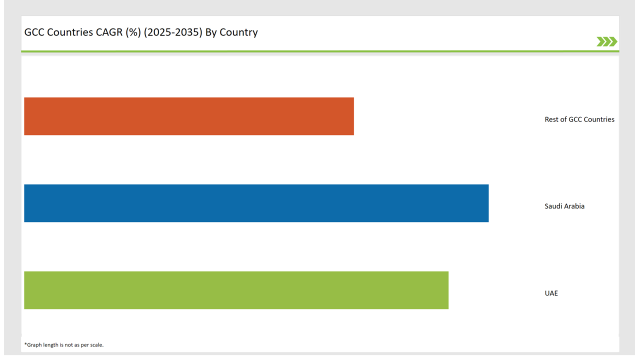
The growth of e-commerce at a rapid pace and changing regulations about labeling are driving the market for Structured Product Label Management in the UAE. ESMA requires digitally compliant solutions, hence increasing the demand for automation. Smart packaging, with its trends of integrating QR code and RFID for traceability, is forcing companies toward structured labeling.
A thriving retail sector, coupled with strict regulations regarding pharmaceuticals and food industries, accelerates market growth. This puts Dubai in the spotlight as an international trade hub, again raising demand for multilingual and regulatory-compliant labeling solutions. Besides, UAE's Smart City Initiatives accelerate digital label transformation across diversified industries.
The GCC SPL management market is highly competitive, with leading technology providers and regulatory consultants investing in AI-driven compliance solutions, blockchain security, and cloud-based regulatory management.
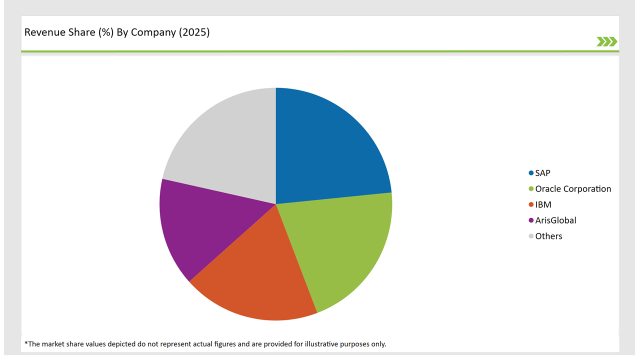
| Vendor | Market Share (2025) |
|---|---|
| SAP | 23.4% |
| Oracle Corporation | 20.8% |
| IBM | 19.2% |
| ArisGlobal | 15.1% |
| Others | 21.5% |
The market is segmented into software and services. Software dominates due to automation and AI-driven compliance tools, while services provide essential regulatory consulting and implementation support.
The industry is categorized into cloud-based and on-premises solutions. Cloud solutions lead due to scalability and accessibility, whereas on-premises solutions remain crucial for organizations prioritizing data security and compliance control.
The market is driven by pharmaceutical companies, biotechnology firms, medical device companies, regulatory authorities, and CROs. Pharmaceutical and biotech firms hold the largest share due to stringent compliance needs and regulatory documentation mandates.
The market will grow at a CAGR of 13.8% from 2025 to 2035.
By 2035, the market will reach USD 12,495.2 million.
Regulatory mandates, cloud-based SPL adoption, AI automation, and blockchain security.
Saudi Arabia and UAE lead due to strong pharmaceutical and biotechnology sector investments.
SAP, Oracle, IBM, and ArisGlobal dominate the SPL management landscape.
| Estimated Size, 2025 | USD 64,983.6 million |
| Projected Size, 2035 | USD 210,576.2 million |
| Value-based CAGR (2025 to 2035) | 12.5% CAGR |
Explore Vertical Solution Insights
View Reports
Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.