The epidural guidance systems market is projected to be worth USD 1.51 billion in 2025. The industry is likely to surpass USD 5.37 billion by 2035 at a CAGR of 13.7% during the projection period.
In 2024, the epidural guidance systems market also grew significantly to an estimated valuation of around USD 1.2 billion. The growth was facilitated by various factors such as rising chronic pain conditions, expansion in the geriatric population, and improvements in healthcare infrastructure. Technical advancements, such as the integration of computer-assisted navigation with real-time imaging modalities like ultrasound and fluoroscopy, improved the accuracy and safety of epidural procedures.
At the regional level, North America continued to be at the forefront, supported by highly developed health systems and a high rate of uptake of medical technology. The United States, specifically, experienced an explosion in the use of ultrasound guidance for epidurals, enhancing precision and patient safety.
The United Kingdom in Europe saw major growth as a result of the increasing application of Point-of-Care Ultrasound (POCUS) for epidural procedures. In contrast, the Asia-Pacific nations, including China and Japan, saw higher adoption levels of AI-based technologies and precise imaging within epidural guidance systems.
Looking towards 2025 and beyond, the epidural guidance systems industry is expected to see further growth. Some major drivers in the market are various technologies continued development, expanding numbers of procedures performed, and greater emphasis on patient safety and outcomes. The convergence of robotics and AI will add further to procedural precision and efficiency. Nevertheless, technical issues like stringent regulatory needs could be a constraint to industry growth.
| Metrics | Values |
|---|---|
| Industry Size (2025E) | USD 1.51 billion |
| Industry Value (2035F) | USD 5.37 billion |
| CAGR (2025 to 2035) | 13.7% |
The epidural guidance systems industry is in a solid growth pattern, fueled by increased demand for accuracy in pain management procedures and technologies like AI-based imaging. Medical providers and medtech firms that provide sophisticated, easy-to-use systems will reap the most benefit, while old device makers and countries with less adoption will be at risk of losing industry share. The industry's momentum is likely to be sustained through 2025 and beyond as patient safety and procedural efficiency become priority concerns.
Speed Up AI-Based Product Development
Make investments in combining AI and real-time imaging technologies in epidural guidance systems to improve procedural accuracy and safety. Invest most in R&D in smart navigation and feedback devices to stand out in a more competitive industry.
Align with Evolving Clinical Protocols and Training Requirements
Partner with healthcare facilities to create training modules and certification programs that reflect new clinical usage of ultrasound and computer-guided procedures. Clinician adoption will fuel industry use and institutional acceptance.
Grow Through Strategic Alliances and M&A
Form distribution alliances in high-growth industries such as Asia-Pacific and make strategic acquisitions of niche imaging technology or robotics firms to extend industry capabilities. Developing an entire solution set will enhance the industry position and global penetration.
| Risk | Probability - Impact |
|---|---|
| Regulatory Changes | High - Significant: Ongoing updates to medical device regulations (e.g., EU MDR, US FDA) could delay product launches or incur high compliance costs. |
| Technological Obsolescence | Medium - High: Rapid technological advancements in imaging and AI could make existing systems obsolete, requiring constant innovation. |
| Supply Chain Disruptions | High - Medium: Material shortages or logistical issues, such as semiconductor shortages, may impact production timelines and costs. |
| Priority | Immediate Action |
|---|---|
| Regulatory Compliance Monitoring | Ensure alignment with evolving regulatory standards (e.g., EU MDR, US FDA) to avoid delays and fines. |
| Technology Upgrades and Innovation | Initiate R&D on AI and machine learning integration for more efficient epidural guidance systems. |
| Supply Chain Diversification | Diversify supplier base for critical components to mitigate risk of delays due to global supply chain disruptions. |
To keep ahead in the developing epidural guidance systems industry, the business has to take regulatory compliance seriously by getting ahead of upcoming standards, especially within the EU and the US. Along with a focus on adopting superior technologies such as AI and automation strategically, this will help keep the business ahead of technology obsolescence.
Secondly, there is a need to diversify the supply chain as well as engage in strong partnerships when it comes to sourcing components. This will assist in reducing the risks of material shortages and production delays. These actions will not only cement the firm's place but also ensure continued growth through innovation, regulatory compliance, and operational resilience. In the future, all these aspects should be at the center of the company's 12-month roadmap.
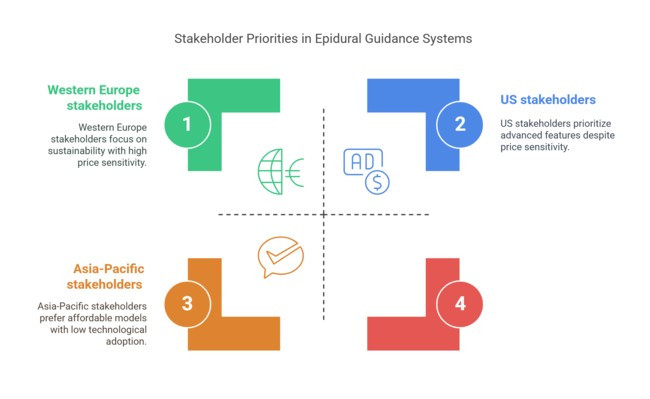
Global Trends:
Regional Variance:
Global Consensus:
Regional Preferences:
Conclusion:
| Countries | Regulatory Impact and Mandatory Certifications |
|---|---|
| United States | The Food and Drug Administration (FDA) regulates epidural guidance systems as medical devices. Manufacturers must obtain FDA approval through the 510(k) industry notification process, demonstrating that the device is safe and effective. Compliance with Quality System Regulation (QSR) standards is also mandatory. |
| India | The Central Drugs Standard Control Organization (CDSCO) oversees medical devices. Epidural guidance systems are classified as Class C devices, requiring an MD-9 manufacturing license. Manufacturers must adhere to the Medical Device Rules, 2017, and ensure compliance with ISO 13485 standards. Importers need an MD-15 import license. |
| European Union | Medical devices are regulated under the EU Medical Device Regulation (MDR). Epidural guidance systems must obtain CE marking by demonstrating compliance with MDR requirements, including clinical evaluations and adherence to ISO 13485 for quality management systems. Notified Bodies assess conformity before industry entry. |
| Japan | The Pharmaceuticals and Medical Devices Agency (PMDA) regulates medical devices. Compliance with the ISO 80369-6 standard for neuraxial applications is mandatory to prevent misconnections. Manufacturers must obtain PMDA approval and ensure adherence to Japan's Pharmaceutical and Medical Device Act (PMD Act). |
| Brazil | The National Health Surveillance Agency (ANVISA) is responsible for medical device regulation. Manufacturers must obtain ANVISA approval, demonstrating compliance with Brazilian Good Manufacturing Practices (BGMP). In June 2024, Milestone Scientific received ANVISA approval to industry its CompuFlo ® Epidural System in Brazil. |
| China | The National Medical Products Administration (NMPA) regulates medical devices. Epidural guidance systems require Class II or III registration, depending on risk classification. Manufacturers must provide clinical data and comply with China’s Good Manufacturing Practices (GMP). |
| Australia | The Therapeutic Goods Administration (TGA) oversees medical devices. Devices must be included in the Australian Register of Therapeutic Goods (ARTG). Compliance with the Essential Principles and demonstration of conformity assessment, often through CE marking or FDA approval, is required. |
| Canada | Health Canada regulates medical devices under the Medical Devices Regulations. Epidural guidance systems are classified as Class II or III devices, necessitating a Medical Device License (MDL). Manufacturers must comply with ISO 13485 and obtain a Medical Device Single Audit Program (MDSAP) certificate. |
| United Kingdom | Post-Brexit, the Medicines and Healthcare Products Regulatory Agency (MHRA) governs medical devices. Devices require UK Conformity Assessed (UKCA) marking. CE marking is recognized until June 2023, after which UKCA marking becomes mandatory. Compliance with UK MDR 2002 regulations is required. |
| South Korea | The Ministry of Food and Drug Safety (MFDS) regulates medical devices. Epidural guidance systems require Class III approval. Manufacturers must submit technical documents, including clinical data, and comply with Korea Good Manufacturing Practice (KGMP) standards. |
Between 2025 and 2035, Image Guidance will be the most profitable technology segment in the epidural guidance systems industry. Growth is being fueled by the greater use of sophisticated imaging methods like fluoroscopy and ultrasound, especially for high-accuracy uses such as spine surgeries, pain relief, and delivery during labor. Image-guided systems offer better safety, lower complication rates, and quicker recovery times, which makes them the better option for both healthcare providers and patients.
Also, the growth of minimally invasive procedures and advancements in real-time imaging technology are adding to the demand. With increasing affordability and adoption, the Image Guidance technology segment is likely to expand with a CAGR of around 8.5% from 2025 to 2035, which would be higher compared to the rate of overall industry growth of 7.3%.
Between 2025 to 2035, the Portable modality segment will be the highest revenue earning, as an increased demand is seen for compact and point-of-care solutions. The portability of epidural guidance systems makes their application feasible in diverse environments, such as ambulatory surgical centers and outpatient clinics, enhancing accessibility and ease for patients. As the trend towards decentralized healthcare continues and cost-saving measures become necessary, portable systems are highly beneficial.
Their application in low-resource settings and their transportation ease in emergency or rural care settings add to the rising interest. With these benefits, the Portable modality segment will grow at a rate of 9.0% CAGR during 2025 to 2035, much greater than the overall industry growth rate.
During 2025 to 2035, Pain Management is likely to be the most profitable application segment. The rising incidence of chronic pain disorders and the worldwide push towards non-opioid pain relief are fueling the use of epidural guidance systems in pain management. Technologies capable of delivering medications with pain-relieving effects more precisely with few side effects are catching a strong tide.
In addition, because medical systems attempt better solutions in handling pain under acute and chronic circumstances, epidural guidance systems promise to solve these problems more efficiently. Demand for minimally invasive pain treatments will propel the growth of this segment, recording an estimated 8.7% CAGR from 2025 to 2035 compared to the growth rate of the total industry.
The Lumbar will remain the highest-value spinal region during 2025 to 2035, primarily driven by the high utilization of epidural injections of the lumbar area and spine surgery. The increasing prevalence of lumbar degenerative diseases, including herniated discs and spinal stenosis, is driving the demand for lumbar epidural guidance systems.
Furthermore, the demand for accurate and safe delivery of treatment in lumbar spine procedures is also on the rise as minimally invasive procedures become more popular. This segment is set to grow by a CAGR of around 8.2% between the years 2025 and 2035 and hence is a prime focus area for investment and growth in the future.
Between the years 2025 and 2035, Hospitals are expected to be the most profitable end-user segment. Hospitals are the main venues where intricate surgeries like spine, thoracic, and orthopedic surgeries are conducted, where epidural guidance systems play a key role.
The increasing number of procedures performed in surgery, as well as the growing emphasis on enhancing patient outcomes, will encourage the use of these systems within hospitals. Also, hospitals will be more likely to embrace newer technologies because of their capacity for higher volumes of procedures and for investing in advanced equipment.
This segment is likely to expand at a CAGR of 8.1% during 2025 to 2035, holding sway in the overall industry.
The United States is the major player in the epidural guidance systems industry, spurred by a large healthcare infrastructure base, high penetration of advanced technologies, and growing demand for minimally invasive treatments.
The increase in chronic pain conditions, as well as developments in imaging modalities and spinal surgeries, will continue to drive the industry forward. A transition to outpatient procedures, including ambulatory surgical centers, will also fuel adoption. Moreover, the increasing demand for user-friendly and portable systems is redefining the industry landscape. The regulatory support and reimbursement policies for innovative medical devices in the U.S. are also driving industry growth.
FMI projects that USA epidural guidance systems sales are likely to rise at a CAGR of 8.5% during the projection period between 2025 and 2035
India's healthcare industry is developing very fast, with rising healthcare access, increasing medical infrastructure, and growing awareness of advanced medical technology. The increasing prevalence of chronic pain disorders and spinal diseases has increased the demand for epidural guidance systems in surgery and pain management.
Furthermore, the growing use of minimally invasive surgeries and increased investment in healthcare infrastructure are likely to fuel growth. Yet, price sensitivity is a challenge the industry faces, with emphasis on cost-saving and low-cost options. As the appeal for portable systems and innovative imaging technology continues to grow, India is likely to emerge as an important industry for epidural guidance systems.
FMI projects that India epidural guidance systems sales are likely to rise at a CAGR of 8.1% during the projection period between 2025 and 2035
With China's growing old age population, high chronic disease incidence, and greater investment in the health sector, China's healthcare industry is rapidly increasing. Pain management, orthopedic, and spinal surgeries will always be in substantial demand in this industry, meaning epidural guidance systems have vast opportunities to expand.
Government efforts and growing private healthcare are encouraging people to adapt more to new technologies. China's healthcare infrastructure is emphasizing modernization and increasing access to advanced medical technology, particularly in tier-2 and tier-3 cities.
FMI projects that China epidural guidance systems sales are likely to rise at a CAGR of 8.3% during the projection period between 2025 and 2035
The UK is experiencing consistent growth in the epidural guidance systems industry, driven by an aging population, increased demand for minimally invasive techniques, and technology innovation in imaging. The National Health Service (NHS) has been working to enhance patient outcomes, promoting greater use of new technologies like ultrasound and fluoroscopy. Additionally, the demand for outpatient procedures and less invasive surgery is further stimulating the expansion of epidural guidance systems.
FMI projects that UK epidural guidance systems sales are likely to rise at a CAGR of 7.8% during the projection period between 2025 and 2035
Germany's healthcare infrastructure is renowned for its cutting-edge medical technologies, and the use of epidural guidance systems is expanding, especially in pain treatment, thoracic, and spinal surgery. Its aging population and increased incidence of chronic diseases are driving the demand for minimally invasive and accurate medical interventions.
The nation's well-established healthcare system, combined with effective reimbursement policies for sophisticated medical devices, is propelling the use of epidural guidance systems. Germany also takes the lead in the use of automation and image-guided systems in surgery.
FMI projects that Germany’s epidural guidance systems sales are likely to rise at a CAGR of 8.2% during the projection period between 2025 and 2035
South Korea is embracing advanced medical technologies at a very fast rate, with the epidural guidance systems industry reaping the rewards of imaging innovations and rising demand for minimally invasive surgical procedures. The healthcare sector is investing heavily in updating infrastructure and delivering high-quality care, which is driving the need for accurate and dependable medical devices.
Since they aim at outpatient care, particularly in ambulatory surgery centers, portable systems are in high demand. Regulatory support and robust economic growth are also anticipated to fuel adoption even further.
FMI projects that South Korea epidural guidance systems sales are likely to rise at a CAGR of 8.0% during the projection period between 2025 and 2035
Japan's aging population and a high prevalence of chronic pain and spinal disorders are major drivers of the epidural guidance systems industry. Increasing demand for sophisticated, non-invasive, and accurate solutions for pain management and spinal surgery is on the rise.
Though Japan traditionally lags behind in adopting new technologies, the demand for minimally invasive and portable equipment is picking up with evolving healthcare requirements and increasing outpatient care focus. Government initiatives aimed at modernizing healthcare in Japan are also driving industry expansion.
FMI projects that Japan epidural guidance systems sales are likely to rise at a CAGR of 7.6% during the projection period between 2025 and 2035
France's healthcare system is established, and its medical device industry is also experiencing robust demand for epidural guidance systems, especially in orthopedic surgeries and pain management. As the number of surgeries increases and minimally invasive methods gain more emphasis, the industry for epidural guidance is predicted to grow even further. France's strong public healthcare system will facilitate the implementation of advanced technologies, and the quest for improved patient outcomes will keep fueling the demand for these systems.
FMI projects that France's epidural guidance systems sales are likely to rise at a CAGR of 7.9% during the projection period between 2025 and 2035
Italy's medical sector is being transformed by moving towards more advanced medical technologies with increased emphasis on patient-centric treatments and minimally invasive techniques. The increasing need for epidural guidance systems for the treatment of chronic pain conditions and spinal pathologies is promoting demand. Cost control challenges for Italy's health sector also exist, but implementation of advanced portable medical devices will increase, especially in outpatient environments.
FMI projects that Italy's epidural guidance systems sales are likely to rise at a CAGR of 7.5% during the projection period between 2025 and 2035
The medical devices and advanced technologies in Australia and New Zealand are developing very fast, with more investments in medical devices and advanced technologies. The epidural guidance systems are in high demand due to the high incidence of spinal conditions, pain management requirements, and the popularity of minimally invasive surgeries. The healthcare infrastructure in Australia is especially strong, with good government support and funding for advanced medical devices. The demand for mobile and stable systems in outpatient clinics is increasing in both nations.
FMI projects that Australia-NZ epidural guidance systems sales are likely to rise at a CAGR of 8.4% during the projection period between 2025 and 2035
The epidural guidance systems industry is moderately consolidated with a dominant few players leveraging technological know-how and robust distribution networks. Competitors are specialized or low-price players. Leadership companies compete in terms of innovations in imaging precision, AI integrations, and robotic-assisted systems, together with strategic relationships with hospitals and research institutions. Pricing approaches differ, with high-end players emphasizing cutting-edge features and others aiming at cost-conscious industries. Growth strategies include expansion in emerging industries and acquisitions to bolster product portfolios.
Key Developments & News (2024)
Ultrasound-Based Systems, Optical Systems, Electromagnetic Systems, Pressure-Based Systems, Traditional Landmark Technique
Hospitals, Ambulatory Surgical Centers, Pain Management Clinics, Academic and Research Institutes
Labor Pain Management, Chronic Pain Management, Surgical Procedures, Diagnostic Procedures
Standalone Systems, Portable/Handheld Systems, Integrated Operating Room Systems
North America, Europe, Asia-Pacific, Latin America, Middle East & Africa
The increasing demand for minimally invasive surgeries and advanced imaging technologies is driving industry growth.
Ultrasound is the most widely used technology due to its non-invasive nature and real-time imaging capabilities.
Key applications include pain management, spine surgery, and orthopedic surgeries.
The USA, China, and India are expected to experience significant growth due to improving healthcare infrastructure and increasing patient demand.
High costs of advanced systems and regulatory hurdles are significant challenges in industry expansion.
Table 01: Global Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Region
Table 02A: Global Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Technology
Table 02B: Global Market Volume (Units) Analysis and Forecast 2012 to 2033, by Technology
Table 03: Global Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Modality
Table 04: Global Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Application
Table 05: Global Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Spinal Region
Table 06: Global Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by End User
Table 07: North America Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Country
Table 08: North America Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Technology
Table 09: North America Market Volume (Units) Analysis and Forecast 2012 to 2033, by Technology
Table 10: North America Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Modality
Table 11: North America Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Application
Table 12: North America Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Spinal Region
Table 13: North America Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by End User
Table 14: Latin America Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Country
Table 15: Latin America Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Technology
Table 16: Latin America Market Volume (Units) Analysis and Forecast 2012 to 2033, by Technology
Table 17: Latin America Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Modality
Table 18: Latin America Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Application
Table 19: Latin America Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Spinal Region
Table 20: Latin America Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by End User
Table 21: Europe Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Country
Table 22: Europe Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Technology
Table 23: Europe Market Volume (Units) Analysis and Forecast 2012 to 2033, by Technology
Table 24: Europe Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Modality
Table 25: Europe Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Application
Table 26: Europe Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Spinal Region
Table 27: Europe Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by End User
Table 28: South Asia Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Country
Table 29: South Asia Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Technology
Table 30: South Asia Market Volume (Units) Analysis and Forecast 2012 to 2033, by Technology
Table 31: South Asia Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Modality
Table 32: South Asia Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Application
Table 33: South Asia Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Spinal Region
Table 34: South Asia Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by End User
Table 35: East Asia Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Country
Table 36: East Asia Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Technology
Table 37: East Asia Market Volume (Units) Analysis and Forecast 2012 to 2033, by Technology
Table 38: East Asia Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Modality
Table 39: East Asia Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Application
Table 40: East Asia Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Spinal Region
Table 41: East Asia Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by End User
Table 42: Oceania Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Country
Table 43: Oceania Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Technology
Table 44: Oceania Market Volume (Units) Analysis and Forecast 2012 to 2033, by Technology
Table 45: Oceania Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Modality
Table 46: Oceania Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Application
Table 47: Oceania Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Spinal Region
Table 48: Oceania Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by End User
Table 49: Middle East & Africa Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Country
Table 50: Middle East & Africa Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Technology
Table 51: Middle East & Africa Market Volume (Units) Analysis and Forecast 2012 to 2033, by Technology
Table 52: Middle East & Africa Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Modality
Table 53: Middle East & Africa Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Application
Table 54: Middle East & Africa Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by Spinal Region
Table 55: Middle East & Africa Market Value (US$ Million) Analysis and Forecast 2012 to 2033, by End User
Figure 01: Global Market Volume (Units), 2012 to 2022
Figure 02: Global Market Volume (Units) & Y-o-Y Growth (%) Analysis, 2023 to 2033
Figure 03: Pricing Analysis per unit (US$), in 2022
Figure 04: Pricing Forecast per unit (US$), in 2033
Figure 05: Global Market Value (US$ Million) Analysis, 2012 to 2022
Figure 06: Global Market Forecast & Y-o-Y Growth, 2023 to 2033
Figure 07: Global Market Absolute $ Opportunity (US$ Million) Analysis, 2022 to 2032
Figure 08: Global Market Value Share (%) Analysis 2023 and 2033, by Technology
Figure 09: Global Market Y-o-Y Growth (%) Analysis 2022 to 2033, by Technology
Figure 10: Global Market Attractiveness Analysis 2023 to 2033, by Technology
Figure 11: Global Market Value Share (%) Analysis 2023 and 2033, by Modality
Figure 12: Global Market Y-o-Y Growth (%) Analysis 2022 to 2033, by Modality
Figure 13: Global Market Attractiveness Analysis 2023 to 2033, by Modality
Figure 14: Global Market Value Share (%) Analysis 2023 and 2033, by Application
Figure 15: Global Market Y-o-Y Growth (%) Analysis 2022 to 2033, by Application
Figure 16: Global Market Attractiveness Analysis 2023 to 2033, by Application
Figure 17: Global Market Value Share (%) Analysis 2023 and 2033, by Spinal Region
Figure 18: Global Market Y-o-Y Growth (%) Analysis 2022 to 2033, by Spinal Region
Figure 19: Global Market Attractiveness Analysis 2023 to 2033, by Spinal Region
Figure 20: Global Market Value Share (%) Analysis 2023 and 2033, by End User
Figure 21: Global Market Y-o-Y Growth (%) Analysis 2022 to 2033, by End User
Figure 22: Global Market Attractiveness Analysis 2023 to 2033, by End User
Figure 23: Global Market Value Share (%) Analysis 2023 and 2033, by Region
Figure 24: Global Market Y-o-Y Growth (%) Analysis 2022 to 2033, by Region
Figure 25: Global Market Attractiveness Analysis 2023 to 2033, by Region
Figure 26: North America Market Value (US$ Million) Analysis, 2012 to 2022
Figure 27: North America Market Value (US$ Million) Forecast, 2022-2032
Figure 28: North America Market Value Share, by Technology (2023 E)
Figure 29: North America Market Value Share, by Modality (2023 E)
Figure 30: North America Market Value Share, by Application (2023 E)
Figure 31: North America Market Value Share, by Spinal Region (2023 E)
Figure 32: North America Market Value Share, by End User (2023 E)
Figure 33: North America Market Value Share, by Country (2023 E)
Figure 34: North America Market Attractiveness Analysis by Technology, 2023 to 2033
Figure 35: North America Market Attractiveness Analysis by Modality, 2023 to 2033
Figure 36: North America Market Attractiveness Analysis by Application, 2023 to 2033
Figure 37: North America Market Attractiveness Analysis by Spinal Region, 2023 to 2033
Figure 38: North America Market Attractiveness Analysis by End User, 2023 to 2033
Figure 39: North America Market Attractiveness Analysis by Country, 2023 to 2033
Figure 40: USA Market Value Proportion Analysis, 2022
Figure 41: Global Vs. USA Growth Comparison
Figure 42: USA Market Share Analysis (%) by Technology, 2023 & 2033
Figure 43: USA Market Share Analysis (%) by Modality, 2023 & 2033
Figure 44: USA Market Share Analysis (%) by Application, 2023 & 2033
Figure 45: USA Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 46: USA Market Share Analysis (%) by End User, 2023 & 2033
Figure 47: Canada Market Value Proportion Analysis, 2022
Figure 48: Global Vs. Canada. Growth Comparison
Figure 49: Canada Market Share Analysis (%) by Technology, 2023 & 2033
Figure 50: Canada Market Share Analysis (%) by Modality, 2023 & 2033
Figure 51: Canada Market Share Analysis (%) by Application, 2023 & 2033
Figure 52: Canada Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 53: Canada Market Share Analysis (%) by End User, 2023 & 2033
Figure 54: Latin America Market Value (US$ Million) Analysis, 2012 to 2022
Figure 55: Latin America Market Value (US$ Million) Forecast, 2022-2032
Figure 56: Latin America Market Value Share, by Technology (2023 E)
Figure 57: Latin America Market Value Share, by Modality (2023 E)
Figure 58: Latin America Market Value Share, by Application (2023 E)
Figure 59: Latin America Market Value Share, by Spinal Region (2023 E)
Figure 60: Latin America Market Value Share, by End User (2023 E)
Figure 61: Latin America Market Value Share, by Country (2023 E)
Figure 62: Latin America Market Attractiveness Analysis by Technology, 2023 to 2033
Figure 63: Latin America Market Attractiveness Analysis by Modality, 2023 to 2033
Figure 64: Latin America Market Attractiveness Analysis by Application, 2023 to 2033
Figure 65: Latin America Market Attractiveness Analysis by Spinal Region, 2023 to 2033
Figure 66: Latin America Market Attractiveness Analysis by End User, 2023 to 2033
Figure 67: Latin America Market Attractiveness Analysis by Country, 2023 to 2033
Figure 68: Mexico Market Value Proportion Analysis, 2022
Figure 69: Global Vs Mexico Growth Comparison
Figure 70: Mexico Market Share Analysis (%) by Technology, 2023 & 2033
Figure 71: Mexico Market Share Analysis (%) by Modality, 2023 & 2033
Figure 72: Mexico Market Share Analysis (%) by Application, 2023 & 2033
Figure 73: Mexico Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 74: Mexico Market Share Analysis (%) by End User, 2023 & 2033
Figure 75: Brazil Market Value Proportion Analysis, 2022
Figure 76: Global Vs. Brazil. Growth Comparison
Figure 77: Brazil Market Share Analysis (%) by Technology, 2023 & 2033
Figure 78: Brazil Market Share Analysis (%) by Modality, 2023 & 2033
Figure 79: Brazil Market Share Analysis (%) by Application, 2023 & 2033
Figure 80: Brazil Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 81: Brazil Market Share Analysis (%) by End User, 2023 & 2033
Figure 82: Argentina Market Value Proportion Analysis, 2022
Figure 83: Global Vs Argentina Growth Comparison
Figure 84: Argentina Market Share Analysis (%) by Technology, 2023 & 2033
Figure 85: Argentina Market Share Analysis (%) by Modality, 2023 & 2033
Figure 86: Argentina Market Share Analysis (%) by Application, 2023 & 2033
Figure 87: Argentina Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 88: Argentina Market Share Analysis (%) by End User, 2023 & 2033
Figure 89: Europe Market Value (US$ Million) Analysis, 2012 to 2022
Figure 90: Europe Market Value (US$ Million) Forecast, 2022-2032
Figure 91: Europe Market Value Share, by Technology (2023 E)
Figure 92: Europe Market Value Share, by Modality (2023 E)
Figure 93: Europe Market Value Share, by Application (2023 E)
Figure 94: Europe Market Value Share, by Spinal Region (2023 E)
Figure 95: Europe Market Value Share, by End User (2023 E)
Figure 96: Europe Market Value Share, by Country (2023 E)
Figure 97: Europe Market Attractiveness Analysis by Technology, 2023 to 2033
Figure 98: Europe Market Attractiveness Analysis by Modality, 2023 to 2033
Figure 99: Europe Market Attractiveness Analysis by Application, 2023 to 2033
Figure 100: Europe Market Attractiveness Analysis by Spinal Region, 2023 to 2033
Figure 101: Europe Market Attractiveness Analysis by End User, 2023 to 2033
Figure 102: Europe Market Attractiveness Analysis by Country, 2023 to 2033
Figure 103: UK Market Value Proportion Analysis, 2022
Figure 104: Global Vs. UK Growth Comparison
Figure 105: UK Market Share Analysis (%) by Technology, 2023 & 2033
Figure 106: UK Market Share Analysis (%) by Modality, 2023 & 2033
Figure 107: UK Market Share Analysis (%) by Application, 2023 & 2033
Figure 108: UK Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 109: UK Market Share Analysis (%) by End User, 2023 & 2033
Figure 110: Germany Market Value Proportion Analysis, 2022
Figure 111: Global Vs. Germany Growth Comparison
Figure 112: Germany Market Share Analysis (%) by Technology, 2023 & 2033
Figure 113: Germany Market Share Analysis (%) by Modality, 2023 & 2033
Figure 114: Germany Market Share Analysis (%) by Application, 2023 & 2033
Figure 115: Germany Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 116: Germany Market Share Analysis (%) by End User, 2023 & 2033
Figure 117: Italy Market Value Proportion Analysis, 2022
Figure 118: Global Vs. Italy Growth Comparison
Figure 119: Italy Market Share Analysis (%) by Technology, 2023 & 2033
Figure 120: Italy Market Share Analysis (%) by Modality, 2023 & 2033
Figure 121: Italy Market Share Analysis (%) by Application, 2023 & 2033
Figure 122: Italy Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 123: Italy Market Share Analysis (%) by End User, 2023 & 2033
Figure 124: France Market Value Proportion Analysis, 2022
Figure 125: Global Vs France Growth Comparison
Figure 126: France Market Share Analysis (%) by Technology, 2023 & 2033
Figure 127: France Market Share Analysis (%) by Modality, 2023 & 2033
Figure 128: France Market Share Analysis (%) by Application, 2023 & 2033
Figure 129: France Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 130: France Market Share Analysis (%) by End User, 2023 & 2033
Figure 131: Spain Market Value Proportion Analysis, 2022
Figure 132: Global Vs Spain Growth Comparison
Figure 133: Spain Market Share Analysis (%) by Technology, 2023 & 2033
Figure 134: Spain Market Share Analysis (%) by Modality, 2023 & 2033
Figure 135: Spain Market Share Analysis (%) by Application, 2023 & 2033
Figure 136: Spain Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 137: Spain Market Share Analysis (%) by End User, 2023 & 2033
Figure 138: Russia Market Value Proportion Analysis, 2022
Figure 139: Global Vs Russia Growth Comparison
Figure 140: Russia Market Share Analysis (%) by Technology, 2023 & 2033
Figure 141: Russia Market Share Analysis (%) by Modality, 2023 & 2033
Figure 142: Russia Market Share Analysis (%) by Application, 2023 & 2033
Figure 143: Russia Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 144: Russia Market Share Analysis (%) by End User, 2023 & 2033
Figure 145: BENELUX Market Value Proportion Analysis, 2022
Figure 146: Global Vs BENELUX Growth Comparison
Figure 147: BENELUX Market Share Analysis (%) by Technology, 2023 & 2033
Figure 148: BENELUX Market Share Analysis (%) by Modality, 2023 & 2033
Figure 149: BENELUX Market Share Analysis (%) by Application, 2023 & 2033
Figure 150: BENELUX Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 151: BENELUX Market Share Analysis (%) by End User, 2023 & 2033
Figure 152: East Asia Market Value (US$ Million) Analysis, 2012 to 2022
Figure 153: East Asia Market Value (US$ Million) Forecast, 2022-2032
Figure 154: East Asia Market Value Share, by Technology (2023 E)
Figure 155: East Asia Market Value Share, by Modality (2023 E)
Figure 156: East Asia Market Value Share, by Application (2023 E)
Figure 157: East Asia Market Value Share, by Spinal Region (2023 E)
Figure 158: East Asia Market Value Share, by End User (2023 E)
Figure 159: East Asia Market Value Share, by Country (2023 E)
Figure 160: East Asia Market Attractiveness Analysis by Technology, 2023 to 2033
Figure 161: East Asia Market Attractiveness Analysis by Modality, 2023 to 2033
Figure 162: East Asia Market Attractiveness Analysis by Application, 2023 to 2033
Figure 163: East Asia Market Attractiveness Analysis by Spinal Region, 2023 to 2033
Figure 164: East Asia Market Attractiveness Analysis by End User, 2023 to 2033
Figure 165: East Asia Market Attractiveness Analysis by Country, 2023 to 2033
Figure 166: China Market Value Proportion Analysis, 2022
Figure 167: Global Vs. China Growth Comparison
Figure 168: China Market Share Analysis (%) by Technology, 2023 & 2033
Figure 169: China Market Share Analysis (%) by Modality, 2023 & 2033
Figure 170: China Market Share Analysis (%) by Application, 2023 & 2033
Figure 171: China Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 172: China Market Share Analysis (%) by End User, 2023 & 2033
Figure 173: Japan Market Value Proportion Analysis, 2022
Figure 174: Global Vs. Japan Growth Comparison
Figure 175: Japan Market Share Analysis (%) by Technology, 2023 & 2033
Figure 176: Japan Market Share Analysis (%) by Modality, 2023 & 2033
Figure 177: Japan Market Share Analysis (%) by Application, 2023 & 2033
Figure 178: Japan Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 179: Japan Market Share Analysis (%) by End User, 2023 & 2033
Figure 180: South Korea Market Value Proportion Analysis, 2022
Figure 181: Global Vs South Korea Growth Comparison
Figure 182: South Korea Market Share Analysis (%) by Technology, 2023 & 2033
Figure 183: South Korea Market Share Analysis (%) by Modality, 2023 & 2033
Figure 184: South Korea Market Share Analysis (%) by Application, 2023 & 2033
Figure 185: South Korea Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 186: South Korea Market Share Analysis (%) by End User, 2023 & 2033
Figure 187: South Asia Market Value (US$ Million) Analysis, 2012 to 2022
Figure 188: South Asia Market Value (US$ Million) Forecast, 2022-2032
Figure 189: South Asia Market Value Share, by Technology (2023 E)
Figure 190: South Asia Market Value Share, by Modality (2023 E)
Figure 191: South Asia Market Value Share, by Application (2023 E)
Figure 192: South Asia Market Value Share, by Spinal Region (2023 E)
Figure 193: South Asia Market Value Share, by End User (2023 E)
Figure 194: South Asia Market Value Share, by Country (2023 E)
Figure 195: South Asia Market Attractiveness Analysis by Technology, 2023 to 2033
Figure 196: South Asia Market Attractiveness Analysis by Modality, 2023 to 2033
Figure 197: South Asia Market Attractiveness Analysis by Application, 2023 to 2033
Figure 198: South Asia Market Attractiveness Analysis by Spinal Region, 2023 to 2033
Figure 199: South Asia Market Attractiveness Analysis by End User, 2023 to 2033
Figure 200: South Asia Market Attractiveness Analysis by Country, 2023 to 2033
Figure 201: India Market Value Proportion Analysis, 2022
Figure 202: Global Vs. India Growth Comparison
Figure 203: India Market Share Analysis (%) by Technology, 2023 & 2033
Figure 204: India Market Share Analysis (%) by Modality, 2023 & 2033
Figure 205: India Market Share Analysis (%) by Application, 2023 & 2033
Figure 206: India Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 207: India Market Share Analysis (%) by End User, 2023 & 2033
Figure 208: Indonesia Market Value Proportion Analysis, 2022
Figure 209: Global Vs. Indonesia Growth Comparison
Figure 210: Indonesia Market Share Analysis (%) by Technology, 2023 & 2033
Figure 211: Indonesia Market Share Analysis (%) by Modality, 2023 & 2033
Figure 212: Indonesia Market Share Analysis (%) by Application, 2023 & 2033
Figure 213: Indonesia Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 214: Indonesia Market Share Analysis (%) by End User, 2023 & 2033
Figure 215: Malaysia Market Value Proportion Analysis, 2022
Figure 216: Global Vs. Malaysia Growth Comparison
Figure 217: Malaysia Market Share Analysis (%) by Technology, 2023 & 2033
Figure 218: Malaysia Market Share Analysis (%) by Modality, 2023 & 2033
Figure 219: Malaysia Market Share Analysis (%) by Application, 2023 & 2033
Figure 220: Malaysia Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 221: Malaysia Market Share Analysis (%) by End User, 2023 & 2033
Figure 222: Thailand Market Value Proportion Analysis, 2022
Figure 223: Global Vs. Thailand Growth Comparison
Figure 224: Thailand Market Share Analysis (%) by Technology, 2023 & 2033
Figure 225: Thailand Market Share Analysis (%) by Modality, 2023 & 2033
Figure 226: Thailand Market Share Analysis (%) by Application, 2023 & 2033
Figure 227: Thailand Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 228: Thailand Market Share Analysis (%) by End User, 2023 & 2033
Figure 229: Oceania Market Value (US$ Million) Analysis, 2012 to 2022
Figure 230: Oceania Market Value (US$ Million) Forecast, 2022-2032
Figure 231: Oceania Market Value Share, by Technology (2023 E)
Figure 232: Oceania Market Value Share, by Modality (2023 E)
Figure 233: Oceania Market Value Share, by Application (2023 E)
Figure 234: Oceania Market Value Share, by Spinal Region (2023 E)
Figure 235: Oceania Market Value Share, by End User (2023 E)
Figure 236: Oceania Market Value Share, by Country (2023 E)
Figure 237: Oceania Market Attractiveness Analysis by Technology, 2023 to 2033
Figure 238: Oceania Market Attractiveness Analysis by Modality, 2023 to 2033
Figure 239: Oceania Market Attractiveness Analysis by Application, 2023 to 2033
Figure 240: Oceania Market Attractiveness Analysis by Spinal Region, 2023 to 2033
Figure 241: Oceania Market Attractiveness Analysis by End User, 2023 to 2033
Figure 242: Oceania Market Attractiveness Analysis by Country, 2023 to 2033
Figure 243: Australia Market Value Proportion Analysis, 2022
Figure 244: Global Vs. Australia Growth Comparison
Figure 245: Australia Market Share Analysis (%) by Technology, 2023 & 2033
Figure 246: Australia Market Share Analysis (%) by Modality, 2023 & 2033
Figure 247: Australia Market Share Analysis (%) by Application, 2023 & 2033
Figure 248: Australia Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 249: Australia Market Share Analysis (%) by End User, 2023 & 2033
Figure 250: New Zealand Market Value Proportion Analysis, 2022
Figure 251: Global Vs New Zealand Growth Comparison
Figure 252: New Zealand Market Share Analysis (%) by Technology, 2023 & 2033
Figure 253: New Zealand Market Share Analysis (%) by Modality, 2023 & 2033
Figure 254: New Zealand Market Share Analysis (%) by Application, 2023 & 2033
Figure 255: New Zealand Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 256: New Zealand Market Share Analysis (%) by End User, 2023 & 2033
Figure 257: Middle East & Africa Market Value (US$ Million) Analysis, 2012 to 2022
Figure 258: Middle East & Africa Market Value (US$ Million) Forecast, 2022-2032
Figure 259: Middle East & Africa Market Value Share, by Technology (2023 E)
Figure 260: Middle East & Africa Market Value Share, by Modality (2023 E)
Figure 261: Middle East & Africa Market Value Share, by Application (2023 E)
Figure 262: Middle East & Africa Market Value Share, by Spinal Region (2023 E)
Figure 263: Middle East & Africa Market Value Share, by End User (2023 E)
Figure 264: Middle East & Africa Market Value Share, by Country (2023 E)
Figure 265: Middle East & Africa Market Attractiveness Analysis by Technology, 2023 to 2033
Figure 266: Middle East & Africa Market Attractiveness Analysis by Modality, 2023 to 2033
Figure 267: Middle East & Africa Market Attractiveness Analysis by Application, 2023 to 2033
Figure 268: Middle East & Africa Market Attractiveness Analysis by Spinal Region, 2023 to 2033
Figure 269: Middle East & Africa Market Attractiveness Analysis by End User, 2023 to 2033
Figure 270: Middle East & Africa Market Attractiveness Analysis by Country, 2023 to 2033
Figure 271: GCC Countries Market Value Proportion Analysis, 2022
Figure 272: Global Vs GCC Countries Growth Comparison
Figure 273: GCC Countries Market Share Analysis (%) by Technology, 2023 & 2033
Figure 274: GCC Countries Market Share Analysis (%) by Modality, 2023 & 2033
Figure 275: GCC Countries Market Share Analysis (%) by Application, 2023 & 2033
Figure 276: GCC Countries Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 277: GCC Countries Market Share Analysis (%) by End User, 2023 & 2033
Figure 278: Türkiye Market Value Proportion Analysis, 2022
Figure 279: Global Vs. Türkiye Growth Comparison
Figure 280: Türkiye Market Share Analysis (%) by Technology, 2023 & 2033
Figure 281: Türkiye Market Share Analysis (%) by Modality, 2023 & 2033
Figure 282: Türkiye Market Share Analysis (%) by Application, 2023 & 2033
Figure 283: Türkiye Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 284: Türkiye Market Share Analysis (%) by End User, 2023 & 2033
Figure 285: South Africa Market Value Proportion Analysis, 2022
Figure 286: Global Vs. South Africa Growth Comparison
Figure 287: South Africa Market Share Analysis (%) by Technology, 2023 & 2033
Figure 288: South Africa Market Share Analysis (%) by Modality, 2023 & 2033
Figure 289: South Africa Market Share Analysis (%) by Application, 2023 & 2033
Figure 290: South Africa Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 291: South Africa Market Share Analysis (%) by End User, 2023 & 2033
Figure 292: Northern Africa Market Value Proportion Analysis, 2022
Figure 293: Global Vs Northern Africa Growth Comparison
Figure 294: Northern Africa Market Share Analysis (%) by Technology, 2023 & 2033
Figure 295: Northern Africa Market Share Analysis (%) by Modality, 2023 & 2033
Figure 296: Northern Africa Market Share Analysis (%) by Application, 2023 & 2033
Figure 297: Northern Africa Market Share Analysis (%) by Spinal Region, 2023 & 2033
Figure 298: Northern Africa Market Share Analysis (%) by End User, 2023 & 2033






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Biopsy Guidance System Market Size and Share Forecast Outlook 2025 to 2035
Autonomous Ultrasound Guidance Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Systems Administration Management Tools Market Size and Share Forecast Outlook 2025 to 2035
VRF Systems Market Growth - Trends & Forecast 2025 to 2035
Cloud Systems Management Software Market Size and Share Forecast Outlook 2025 to 2035
Hi-Fi Systems Market Size and Share Forecast Outlook 2025 to 2035
Cough systems Market
Backpack Systems Market Size and Share Forecast Outlook 2025 to 2035
Unmanned Systems Market Analysis - Size, Share, & Forecast Outlook 2025 to 2035
DC Power Systems Market Trends - Growth, Demand & Forecast 2025 to 2035
Catheter Systems Market
Reporter Systems Market
Aerostat Systems Market
Cryogenic Systems Market Size and Share Forecast Outlook 2025 to 2035
Air Brake Systems Market Growth & Demand 2025 to 2035
Metrology Systems Market
Fluid Bed Systems Market
Cognitive Systems Spending Market Report – Growth & Forecast 2016-2026
Nurse Call Systems Market Insights - Size, Share & Forecast 2025 to 2035
Excitation Systems Market Analysis – Growth, Demand & Forecast 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA