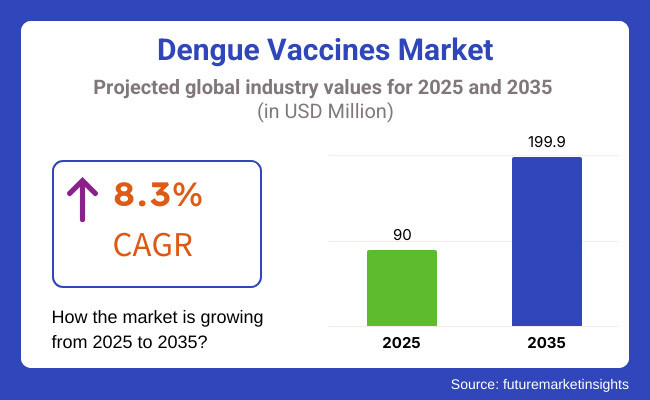
The global Dengue Vaccines Market is expected to be reach USD 90.0 million in 2025 and is likely to expand up to approximately USD 199.9 million by the end of 2035. The sales are believed to rise with a CAGR of 8.3% during the period of 2025 to 2035.
The dengue vaccine market is set for strong growth driven by rising cases of dengue fever, climate change and urbanization. Reported cases of dengue globally reached a record 13.0 million in 2024, nearly doubling that of 2023 emphasizing the need for vaccination. Climate change is among the main drivers coupled with expanding mosquito breeding grounds, increasing levels of transmission, and bringing dengue to new regions.
Tropical and subtropical areas are undergoing rapid urbanization which is likely to have conducive breeding conditions for mosquitoes and thereby increasing infection risk. Government organization are actively incorporating dengue vaccines into national immunization schedules across different countries.
The launch of new vaccines Qdenga from Tadeka Pharmaceuticals offering protection against all four serotypes of dengue is also fuelling market demand even further. Government educational campaigns are also fuelling the vaccination, and further developments in biotechnology will continue to improve the vaccines.
With positive government support, advancements in technology, and growing awareness, the dengue vaccine market will develop exponentially. All these factors together promise a stable future, reducing hospitalizations due to dengue and the disease burden across the globe.
Brazil, Peru, and Argentina have the highest number of dengue cases in Latin America. The tropical climate in this region is the main cause for the consistent cases of dengue. Urbanization and the lack of effective vector control methods to curb these diseases are also factors for the mosquito infestation that leads to an outbreak.
In 2024, Brazil and Argentina experienced a remarkable increase in dengue, which made the situation so bad that the government had to intervene quickly. For instance, at the start of March 2024, Argentina included Qdenga (TAK-003) along with other vaccines that are given to children in the national vaccination scheme in a bid to fight the rising public health threat due to dengue.
The government has taken the initiative by involving vaccine manufacturers in the process, especially in city centers where there is an abundance of mosquito breeding sites. Colombia also have acknowledged the significance of immunizing people against dengue, hence emphasizing communities that are more at risk. Governments are taking steps to fund vector control and mosquito eradication programs as well.
They have introduced public-private collaborations to ensure that vaccines are available for citizens. The public became more informed through educational campaigns that were put up to drive vaccine uptake. With the aid of additional funding and expanded programs, Latin America remains a major player in the dengue vaccine market for the near future.
Dengue cases in Europe is also being faced with a similar challenge as fuelled by climate change and worldwide movements of people across different countries rising infection cases. Normally, Europe was absolutely free of dengue, however, the weather has become more favourable to it and the number of mosquito populations has also increased over the years which is a cause of disease epidemics.
Countries like UK, Germany, and France have already detected local dengue transmission, which is something wholly unprecedented for the aforesaid countries. The recent years have seen that the strain of dengue fever on the European continent has been increasing hence the introduction of the vaccination strategies.
The European Medicines Agency allowed for the release of Qdenga vaccine to the people in those European countries that would like to have it. Governments are focusing on the voracious population of dengue vaccine among the travellers, expatriates and the inhabitants of those areas that are getting warmer due to climate change.
Tourists who are traveling to places that have a high number of dengue cases are also partially responsible for the rise in the number of pre-travel vaccines that people have to get. European states have allocated budget for research on mosquito control and dengue prevention. Climate change adaptation has added dengue fever to the list of possible risks, and therefore it has become a public health issue.
The expansion of vaccination, coupled with the rise in awareness for travelers and government-driven health programs, will create the right business environment for the dengue vaccine market in Europe.
Asia-Pacific is the only region where the demand and supply of dengue vaccines are on rise as these countries such as India, Indonesia, Thailand and the Philippines have a tropical wet and dry climate and dengue outbreaks are more prevalent. Almost half of the cases of dengue worldwide are from this region, making vaccination an important factor in the overall management.
Indonesia and Thailand have received the go-ahead to use Qdenga in 2023 along with Dengvaxia which are already in the market. Dengvaxia is approved in Singapore and the country is focussing more towards approval of Qdenga.
India has to deal with substantial reported cases of dengue which reaches a few millions annually, has ploughed a lot of money into vaccine manufacturing or centrally acquired vaccination plans. The country is also consolidating public-private partnerships to facilitate vaccine supply and have better access.
Urbanization, climate change and poor waste management along with mosquito control are some of the foremost factors for the outspread of dengue that led to the governmental sector pushing for more allocations in mosquito eradication programs.
The increase in healthcare services, both in number and awareness for the vaccine, has led to the furthering of immunization programs. With continuous progress in the development of vaccines and the growing scope of immunization schemes, Asia-Pacific is bound to be a leader in the global market of dengue vaccines and will further expand in the following years.
Challenges
The market for the dengue vaccine faces a number of hurdles that limit widespread adoption. Dengue virus are fairly complicated with four serotypes which complicates vaccine production. An effective vaccine has to give equal protection against all four serotypes to avoid severe disease in people with previous infections.
Previous vaccines have yielded mixed outcomes, leading to reluctance among healthcare workers and the general public. Regulatory authorities mandate strict approval procedures, delaying access to new markets for the vaccines. Companies struggling with excessive production expenses and cold-chain distribution requirements, hindering reach into low and middle-income countries.
Misinformation and past controversies about vaccines discourage further public uptake. Vaccines manufacturers need to counter these issues by investing in awareness campaigns. Government should play a pivotal role in maintaining vaccine cost, and enforcing stringent post-market surveillance. Increasing access to vaccines will boost public confidence and will drive dengue vaccine demand and avoiding future outbreaks.
Opportunities
The market for dengue vaccine is huge with vast growth potential, driven by rising cases of dengue and heavy government backing. As the disease burden expanded in Asia-Pacific and Latin America, there has given rise to a high demand for effective vaccine manufacturing. Key players are extending their reach to emerging nations where dengue continues to be an important public health risk.
With technology advancement, scientists are able to create future generation vaccines with enhanced efficacy and wider protection. Governments and health authorities continue to fund mass immunization campaigns which will have a significant uptake in the forecast years.
Public-private collaborations fortify research, manufacture, and supply, so vaccines are distributed to vulnerable populations. Increased education about dengue prevention and better healthcare facilities encourage vaccine uptake.
Health authorities can establish trust among the population by educating and being open about the facts, fostering long-term immunization practice. By taking advantage of these opportunities, stakeholders can propel the dengue vaccine market and make vaccination a mainstay in global dengue prevention.
Between 2020 and 2024, Dengue vaccine market has undergone significant transformation since the cases of dengue are increasing, new vaccines have been licensed and the regulations have been revised in terms of its form. The endemic nations were the ones who helped government agencies to make the arsenal stronger against dengue, which in turn led to the emergence of the vaccination programs.
The European Medicines Agency's (EMA) approval of Qdenga (TAK-003) in 2022 and its follow-up adoption throughout countries brought about the vaccine being ubiquitous. Qdenga not only had a simpler vaccine regimen than Dengvaxia, which required antivaccine screening, but it also protected a larger portion of the population from dengue virus infection.
Health authorities orchestrated the inclusion of the dengue vaccine in national immunization programs including Argentina's great step forward in 2024 when Qdenga was added to the list. The manufacturers were successful in adding the stability and storage conditions improvements which were the two biggest obstacles to ensuring better distribution around the low-resource areas.
Nevertheless, they were hampered by various challenges such as community doubts about immunization, high production costs, and the need for cold-chain distribution, which resulted in the slow uptake of the vaccines.
Between 2025 and 2035, the vaccine manufacturers will be launching the modern dengue vaccines with better efficacy, prolonged immunity, and superior serotype protection. Biotechnologists will be the ones researching mRNA vaccine technology, utilizing transformative techniques for COVID-19 vaccination.
Governments will put into action universal dengue vaccination campaigns in hot spot areas as well as increasing public moneys and extending immunization schedules. Furthermore, countries will add the dengue vaccine to their routine childhood immunization schedules as with the case of measles and polio vaccines.
The biotechnology sector will be the frontiers in designing vaccines that do not require refrigeration, thereby, not only making logistics easier but facilitating the provision of vaccines in isolated areas.
The more favorable ecological conditions insects will encounter due to climate change, and consequently, the dengue virus will be transmitted to areas that were newly infected, resulting in the rise of vaccine demand in Southern Europe and North America.
Market Shifts: A Comparative Analysis (2020 to 2024 vs. 2025 to 2035)
| Market Shift | 2020 to 2024 |
|---|---|
| Regulatory Landscape | Safety and efficacy came first, guided by authorities with region-based rules. The approval of Qdenga increased regulatory acceptability. |
| Technological Advancements | New formulations enhanced the immune response and broadened serotype protection. Stability-enhancing innovations facilitated better storage and handling |
| Consumer Demand | Increased need in endemic countries, national immunization schemes enlarged. Dengvaxia hesitation reduced demand; Qdenga enhanced acceptability. |
| Market Growth Drivers | Increased case occurrences of dengue fever and WHO-sponsored initiatives raised vaccination demand. Private-public partnerships enhanced development and accessibility of vaccines. |
| Sustainability | First steps towards green packaging and recycling of vaccine vials. Some producers applied efficient energy production. |
| Supply Chain Dynamics | Conventional vaccine distribution was through wholesalers and government authorities with minimal growth of online procurement. |
| Market Shift | 2025 to 2035 |
|---|---|
| Regulatory Landscape | Stronger post-market surveillance, digital monitoring, and more rapid approval of next-generation dengue vaccines for high-risk areas |
| Technological Advancements | mRNA dengue vaccines, thermostable vaccine formulations, and AI-generated outbreak predictions will enhance vaccine distribution and deployment. |
| Consumer Demand | Increasing awareness among people, increased tourism requirements, and national immunization childhood programs will enhance greater uptake. |
| Market Growth Drivers | Increased government budget and climate change, wider expansion of dengue cases, and wider reach of government schemes will spur greater growth |
| Sustainability | Biodegradable packaging, efficient production, and environmentally friendly cold storage will shape future vaccine production. |
| Supply Chain Dynamics | Blockchain tracking, live monitoring, and responsible material sourcing will guarantee fair vaccine distribution. |
The dengue vaccines have a potential for enormous growth due to continuous development of next-generation vaccine technologies, the expansion of immunization programs, and the unchanging need for economic options for dengue prevention. Increased government spending, the effect of climate change on the spread of the virus, and the availability of more vaccines will be the main drivers for the market's constant growth.
Brazil has touched a peak on the global dengue infection rates. The country witnessed a considerable uptick in cases in 2024, resulting in the authorities launching mass medication initiatives. The government is making Qdenga more available in the high-risk areas and the cities that have a lot of mosquito breeding sites.
The Ministry of Health reported Brazil has made public-private partnerships more effective by ensuring vaccine affordability and speeding up vaccination. The issues such as public hesitance on vaccines and problems with distribution of the vaccines in the rural areas continue to exist.
Nonetheless, by allocating more funds and launching robust public health campaigns, Brazil is being anticipated to take the lead in the Dengue vaccine uptake in the Latin America region.
Market Forecast
| Country | CAGR (2025 to 2035) |
|---|---|
| Brazil | 7.6% |
Rising International travel and climate change have put dengue prevention in the spotlight even though UK is not a dengue-endemic country. The advent of Qdenga has allowed pre-travel vaccination to be more widely utilized, especially for those traveling to Asia-Pacific and Latin America.
The NHS and private practitioners are disseminating information on dengue prevention through travel alert programs. Aside from that, there are scientific research institutes based in the UK looking into the migration of mosquitoes due to climate change, which would aid in the prediction of possible future outbreaks.
By the way, the government is trying to ensure pre-travel immunization is well implemented and is still sustaining the funding of tropical disease research... All this puts the UK apart as a lead in the fight against dengue, especially in the case of vaccines.
Market Forecast
| Country | CAGR (2025 to 2035) |
|---|---|
| UK | 7.1% |
The dengue landscape of France has been changing in the past time, with ever more locally infected cases of the disease in the southern parts of the country. The increase in average temperatures and the size of the mosquito populations have raised the possibility of dengue outbreak, which resulted in the government-organized programs for mosquito surveillance.
The Qdenga supply for travelers has also been increased, covering the risk for the people who travel to the endemic areas. Also, in the overseas territories like Guadeloupe and Réunion, high dengue transmission is still an ongoing issue, which forces the government to make vaccines more accessible and take care of the public health sector.
The increase of the Visa Kitchen project is juxtaposed with the need for the French government to prioritize the prevention of thede dengue disease, conducting research on mosquito control and vaccination programs to mitigate the future outbreaks.
Market Forecast
| Country | CAGR (2025 to 2035) |
|---|---|
| France | 5.1% |
Indonesia is the most dengue-affected nation experiencing recurrent outbreaks each year. The government has authorized Qdenga and launched massive vaccination activities to reduce the infection rate, mainly in the highly populated cities of Jakarta, Surabaya, and Medan.
Through public health campaigns, people are learning the preventive measures against dengue, and newly formed partnerships with global health organizations are facilitating the increase in vaccine manufacturing capacity. In a bid to control the transmission of dengue fever, the government of Brazil made a move by administering Qdenga to counties deemed severe and the likely urban centers where the breeding of mosquitoes is widespread.
To add, the Ministry of Health of Brazil is emphasizing public-private partnerships to make vaccines less expensive and to hasten the vaccination process. Nevertheless, the biggest hurdles are the issues of vaccine reluctance and lack of distribution in remote territories.
On account of the unyielding government funding and successful public health operations, Brazil is poised to become the front-runner in the region in relation to extensive use of the dengue vaccine.
Market Forecast
| Country | CAGR (2025 to 2035) |
|---|---|
| Indonesia | 10.2% |
Thailand is still struggling with significant dengue cases as a seasonal epidemic, where the number of cases is highest during the monsoon season. With the launch of new vaccine in the country, the government is actively promoting vaccine distribution, particularly in areas of high transmission rates in Bangkok, Phuket and Chiang Mai.
With tourism booming in Thailand, it has also spurred demand for vaccines, with the authorities encouraging tourists and expatriates to be vaccinated prior to traveling to dengue-endemic destinations. Thai research institutions are also working together with multinational pharmaceutical companies in coming up with improved dengue vaccines that will provide long-term prevention.
There is government support and aggressive effort towards vector management, and as a result, Thailand is rapidly becoming a pace-setter of dengue vaccine application within Southeast Asia.
Market Forecast
| Country | CAGR (2025 to 2035) |
|---|---|
| Thailand | 13.1% |
Niche Markets and Targeted Immunization to Drive Dengvaxia Vaccine Growth
Dengvaxia is the first dengue vaccine developed by Sanofi. It was the first licensed dengue vaccine and it suffered considerable hurdles in the market owing to the safety concerns. The regulatory authorities imposed restrictions on its use, allowing only those individuals who had been previously infected with the dengue virus to receive the vaccine.
This vaccines is still a potential solution in certain high-burden regions, especially in areas where a pre-vaccination testing facility is available. But its sale has been slower in last couple of years with the shift towards newer vaccines like Qdenga.
In certain travel health sectors and endemic areas, where pre-screening is feasible, the vaccine still holds market relevance. Due to the preference of wider-eligibility vaccines by governmental and healthcare providers, the future earnings of Sanofi are expected to be slower across different countries.
Broad Accessibility and Expanding Global Adoption Driving Qdenga Sales
Qdenga which was developed by Takeda Pharmaceuticals, has taken off at a breathtaking speed owing to the eligibility for all ages since its approval by the European Medicines Agency (EMA) in 2022. Qdenga is unlike Dengvaxia since it does not need to conduct a test on the previous dengue infection, and thus is easier and more widely used for the mass vaccination.
The countries with the heaviest dengue burden, including Brazil, Argentina, Indonesia, and Thailand, have already given their endorsement and have added the Qdenga vaccine to their national vaccination programs, which are compulsory once signed. This has led to a considerable increase in the uptake of the program.
The market expansion of Qdenga is expected to keep on growing with the government contracts, the adoption of private health care, and the vaccinations which are travel-related. Due to the climate change and the increase of the urban population, there will be a steady demand for quality vaccines. Takeda is steering towards attaining more approvals in these territories. Qdenga is strategized to take over the market in the next decade.
Hospital Segment to Drive the Sales of Dengue Vaccines
Hospitals actively drive dengue vaccine adoption by serving as primary immunization centers in both endemic and non-endemic regions. Healthcare providers administer vaccines as part of routine immunization programs, particularly targeting high-risk groups such as children, the elderly, and immunocompromised individuals. In dengue-endemic areas, hospitals integrate dengue vaccines into preventive care initiatives, ensuring widespread access.
Healthcare professionals conduct pre-screening and monitor post-vaccination responses, making hospitals a preferred setting for Dengvaxia, which requires prior dengue exposure confirmation. Hospitals also promote the sales of Qdenga due to its broad eligibility, increasing patient uptake. Traveller immunization clinics in hospitals offer dengue vaccines to individuals visiting high-risk regions, further boosting demand.
With governments expanding national dengue vaccination programs, hospitals will enhance vaccine distribution, increase accessibility, and improve public confidence. As global dengue cases continue to rise, hospitals will play a pivotal role in large-scale dengue immunization, strengthening the overall vaccine market.
The Dengue Vaccines Market is highly competitive, driven by increasing dengue cases across different countries. Manufacturers are investing in developing cost-effective solutions vaccines to maintain a competitive edge.
Market Share Analysis by Company
| Company Name | Estimated Market Share (%) |
|---|---|
| Sanofi | 5-10% |
| Takeda Pharmaceuticals | 90-95% |
| Company Name | Key Offerings/Activities |
|---|---|
| Sanofi (Dengvaxia - CYD-TDV) | First-ever approved dengue vaccine, primarily recommended for individuals with prior dengue exposure. Requires pre-screening before administration. Available in select endemic regions with regulatory restrictions. |
| Takeda Pharmaceuticals (Qdenga - TAK-003) | New-generation dengue vaccine approved for broad population use, without the need for pre-screening. Rapidly expanding global market presence with approvals in multiple countries, including Latin America, Asia-Pacific, and Europe. |
Key Company Insights
Beyond the leading companies, several other manufacturers contribute significantly to the market, enhancing product advancements in the clinical trials. These include:
These companies focus on expanding the reach of NPWT solutions, offering competitive pricing and cutting-edge innovations to meet diverse healthcare provider and patient needs.
The global Dengue Vaccines industry is projected to witness CAGR of 8.3% between 2025 and 2035.
The global Dengue Vaccines industry stood at USD 84.3 million in 2024.
The global Dengue Vaccines industry is anticipated to reach USD 199.9 million by 2035 end.
China is expected to show a CAGR of 8.2% in the assessment period.
The key players operating in the global Dengue Vaccines industry are Tadeka Pharmaceuticals and Sanofi.
Table 1: Global Market Value (US$ Million) Forecast by Region, 2018 to 2033
Table 2: Global Market Volume (Units) Forecast by Region, 2018 to 2033
Table 3: Global Market Value (US$ Million) Forecast by Product, 2018 to 2033
Table 4: Global Market Volume (Units) Forecast by Product, 2018 to 2033
Table 5: Global Market Value (US$ Million) Forecast by End-User, 2018 to 2033
Table 6: Global Market Volume (Units) Forecast by End-User, 2018 to 2033
Table 7: North America Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 8: North America Market Volume (Units) Forecast by Country, 2018 to 2033
Table 9: North America Market Value (US$ Million) Forecast by Product, 2018 to 2033
Table 10: North America Market Volume (Units) Forecast by Product, 2018 to 2033
Table 11: North America Market Value (US$ Million) Forecast by End-User, 2018 to 2033
Table 12: North America Market Volume (Units) Forecast by End-User, 2018 to 2033
Table 13: Latin America Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 14: Latin America Market Volume (Units) Forecast by Country, 2018 to 2033
Table 15: Latin America Market Value (US$ Million) Forecast by Product, 2018 to 2033
Table 16: Latin America Market Volume (Units) Forecast by Product, 2018 to 2033
Table 17: Latin America Market Value (US$ Million) Forecast by End-User, 2018 to 2033
Table 18: Latin America Market Volume (Units) Forecast by End-User, 2018 to 2033
Table 19: Europe Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 20: Europe Market Volume (Units) Forecast by Country, 2018 to 2033
Table 21: Europe Market Value (US$ Million) Forecast by Product, 2018 to 2033
Table 22: Europe Market Volume (Units) Forecast by Product, 2018 to 2033
Table 23: Europe Market Value (US$ Million) Forecast by End-User, 2018 to 2033
Table 24: Europe Market Volume (Units) Forecast by End-User, 2018 to 2033
Table 25: Asia Pacific Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 26: Asia Pacific Market Volume (Units) Forecast by Country, 2018 to 2033
Table 27: Asia Pacific Market Value (US$ Million) Forecast by Product, 2018 to 2033
Table 28: Asia Pacific Market Volume (Units) Forecast by Product, 2018 to 2033
Table 29: Asia Pacific Market Value (US$ Million) Forecast by End-User, 2018 to 2033
Table 30: Asia Pacific Market Volume (Units) Forecast by End-User, 2018 to 2033
Table 31: MEA Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 32: MEA Market Volume (Units) Forecast by Country, 2018 to 2033
Table 33: MEA Market Value (US$ Million) Forecast by Product, 2018 to 2033
Table 34: MEA Market Volume (Units) Forecast by Product, 2018 to 2033
Table 35: MEA Market Value (US$ Million) Forecast by End-User, 2018 to 2033
Table 36: MEA Market Volume (Units) Forecast by End-User, 2018 to 2033
Figure 1: Global Market Value (US$ Million) by Product, 2023 to 2033
Figure 2: Global Market Value (US$ Million) by End-User, 2023 to 2033
Figure 3: Global Market Value (US$ Million) by Region, 2023 to 2033
Figure 4: Global Market Value (US$ Million) Analysis by Region, 2018 to 2033
Figure 5: Global Market Volume (Units) Analysis by Region, 2018 to 2033
Figure 6: Global Market Value Share (%) and BPS Analysis by Region, 2023 to 2033
Figure 7: Global Market Y-o-Y Growth (%) Projections by Region, 2023 to 2033
Figure 8: Global Market Value (US$ Million) Analysis by Product, 2018 to 2033
Figure 9: Global Market Volume (Units) Analysis by Product, 2018 to 2033
Figure 10: Global Market Value Share (%) and BPS Analysis by Product, 2023 to 2033
Figure 11: Global Market Y-o-Y Growth (%) Projections by Product, 2023 to 2033
Figure 12: Global Market Value (US$ Million) Analysis by End-User, 2018 to 2033
Figure 13: Global Market Volume (Units) Analysis by End-User, 2018 to 2033
Figure 14: Global Market Value Share (%) and BPS Analysis by End-User, 2023 to 2033
Figure 15: Global Market Y-o-Y Growth (%) Projections by End-User, 2023 to 2033
Figure 16: Global Market Attractiveness by Product, 2023 to 2033
Figure 17: Global Market Attractiveness by End-User, 2023 to 2033
Figure 18: Global Market Attractiveness by Region, 2023 to 2033
Figure 19: North America Market Value (US$ Million) by Product, 2023 to 2033
Figure 20: North America Market Value (US$ Million) by End-User, 2023 to 2033
Figure 21: North America Market Value (US$ Million) by Country, 2023 to 2033
Figure 22: North America Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 23: North America Market Volume (Units) Analysis by Country, 2018 to 2033
Figure 24: North America Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 25: North America Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 26: North America Market Value (US$ Million) Analysis by Product, 2018 to 2033
Figure 27: North America Market Volume (Units) Analysis by Product, 2018 to 2033
Figure 28: North America Market Value Share (%) and BPS Analysis by Product, 2023 to 2033
Figure 29: North America Market Y-o-Y Growth (%) Projections by Product, 2023 to 2033
Figure 30: North America Market Value (US$ Million) Analysis by End-User, 2018 to 2033
Figure 31: North America Market Volume (Units) Analysis by End-User, 2018 to 2033
Figure 32: North America Market Value Share (%) and BPS Analysis by End-User, 2023 to 2033
Figure 33: North America Market Y-o-Y Growth (%) Projections by End-User, 2023 to 2033
Figure 34: North America Market Attractiveness by Product, 2023 to 2033
Figure 35: North America Market Attractiveness by End-User, 2023 to 2033
Figure 36: North America Market Attractiveness by Country, 2023 to 2033
Figure 37: Latin America Market Value (US$ Million) by Product, 2023 to 2033
Figure 38: Latin America Market Value (US$ Million) by End-User, 2023 to 2033
Figure 39: Latin America Market Value (US$ Million) by Country, 2023 to 2033
Figure 40: Latin America Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 41: Latin America Market Volume (Units) Analysis by Country, 2018 to 2033
Figure 42: Latin America Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 43: Latin America Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 44: Latin America Market Value (US$ Million) Analysis by Product, 2018 to 2033
Figure 45: Latin America Market Volume (Units) Analysis by Product, 2018 to 2033
Figure 46: Latin America Market Value Share (%) and BPS Analysis by Product, 2023 to 2033
Figure 47: Latin America Market Y-o-Y Growth (%) Projections by Product, 2023 to 2033
Figure 48: Latin America Market Value (US$ Million) Analysis by End-User, 2018 to 2033
Figure 49: Latin America Market Volume (Units) Analysis by End-User, 2018 to 2033
Figure 50: Latin America Market Value Share (%) and BPS Analysis by End-User, 2023 to 2033
Figure 51: Latin America Market Y-o-Y Growth (%) Projections by End-User, 2023 to 2033
Figure 52: Latin America Market Attractiveness by Product, 2023 to 2033
Figure 53: Latin America Market Attractiveness by End-User, 2023 to 2033
Figure 54: Latin America Market Attractiveness by Country, 2023 to 2033
Figure 55: Europe Market Value (US$ Million) by Product, 2023 to 2033
Figure 56: Europe Market Value (US$ Million) by End-User, 2023 to 2033
Figure 57: Europe Market Value (US$ Million) by Country, 2023 to 2033
Figure 58: Europe Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 59: Europe Market Volume (Units) Analysis by Country, 2018 to 2033
Figure 60: Europe Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 61: Europe Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 62: Europe Market Value (US$ Million) Analysis by Product, 2018 to 2033
Figure 63: Europe Market Volume (Units) Analysis by Product, 2018 to 2033
Figure 64: Europe Market Value Share (%) and BPS Analysis by Product, 2023 to 2033
Figure 65: Europe Market Y-o-Y Growth (%) Projections by Product, 2023 to 2033
Figure 66: Europe Market Value (US$ Million) Analysis by End-User, 2018 to 2033
Figure 67: Europe Market Volume (Units) Analysis by End-User, 2018 to 2033
Figure 68: Europe Market Value Share (%) and BPS Analysis by End-User, 2023 to 2033
Figure 69: Europe Market Y-o-Y Growth (%) Projections by End-User, 2023 to 2033
Figure 70: Europe Market Attractiveness by Product, 2023 to 2033
Figure 71: Europe Market Attractiveness by End-User, 2023 to 2033
Figure 72: Europe Market Attractiveness by Country, 2023 to 2033
Figure 73: Asia Pacific Market Value (US$ Million) by Product, 2023 to 2033
Figure 74: Asia Pacific Market Value (US$ Million) by End-User, 2023 to 2033
Figure 75: Asia Pacific Market Value (US$ Million) by Country, 2023 to 2033
Figure 76: Asia Pacific Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 77: Asia Pacific Market Volume (Units) Analysis by Country, 2018 to 2033
Figure 78: Asia Pacific Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 79: Asia Pacific Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 80: Asia Pacific Market Value (US$ Million) Analysis by Product, 2018 to 2033
Figure 81: Asia Pacific Market Volume (Units) Analysis by Product, 2018 to 2033
Figure 82: Asia Pacific Market Value Share (%) and BPS Analysis by Product, 2023 to 2033
Figure 83: Asia Pacific Market Y-o-Y Growth (%) Projections by Product, 2023 to 2033
Figure 84: Asia Pacific Market Value (US$ Million) Analysis by End-User, 2018 to 2033
Figure 85: Asia Pacific Market Volume (Units) Analysis by End-User, 2018 to 2033
Figure 86: Asia Pacific Market Value Share (%) and BPS Analysis by End-User, 2023 to 2033
Figure 87: Asia Pacific Market Y-o-Y Growth (%) Projections by End-User, 2023 to 2033
Figure 88: Asia Pacific Market Attractiveness by Product, 2023 to 2033
Figure 89: Asia Pacific Market Attractiveness by End-User, 2023 to 2033
Figure 90: Asia Pacific Market Attractiveness by Country, 2023 to 2033
Figure 91: MEA Market Value (US$ Million) by Product, 2023 to 2033
Figure 92: MEA Market Value (US$ Million) by End-User, 2023 to 2033
Figure 93: MEA Market Value (US$ Million) by Country, 2023 to 2033
Figure 94: MEA Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 95: MEA Market Volume (Units) Analysis by Country, 2018 to 2033
Figure 96: MEA Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 97: MEA Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 98: MEA Market Value (US$ Million) Analysis by Product, 2018 to 2033
Figure 99: MEA Market Volume (Units) Analysis by Product, 2018 to 2033
Figure 100: MEA Market Value Share (%) and BPS Analysis by Product, 2023 to 2033
Figure 101: MEA Market Y-o-Y Growth (%) Projections by Product, 2023 to 2033
Figure 102: MEA Market Value (US$ Million) Analysis by End-User, 2018 to 2033
Figure 103: MEA Market Volume (Units) Analysis by End-User, 2018 to 2033
Figure 104: MEA Market Value Share (%) and BPS Analysis by End-User, 2023 to 2033
Figure 105: MEA Market Y-o-Y Growth (%) Projections by End-User, 2023 to 2033
Figure 106: MEA Market Attractiveness by Product, 2023 to 2033
Figure 107: MEA Market Attractiveness by End-User, 2023 to 2033
Figure 108: MEA Market Attractiveness by Country, 2023 to 2033






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Dengue Fever Treatment Market - Growth & Therapeutic Innovations 2025 to 2035
Vaccines Market Insights - Trends, Growth & Forecast 2025 to 2035
Cat Vaccines Market Size and Share Forecast Outlook 2025 to 2035
Fish Vaccines Market
Live Vaccines Market
Nasal vaccines Market
Travel Vaccines Market Size and Share Forecast Outlook 2025 to 2035
Cancer Vaccines Market Analysis by Technology, Treatment Method, Application and Region from 2025 to 2035
Varicella Vaccines Market Insights - Growth, Trends & Forecast 2024 to 2034
Veterinary Vaccines Market Analysis - Size, Share, and Forecast 2025 to 2035
Attenuated Vaccines Market Analysis - Size, Share & Forecast 2024 to 2034
Aquaculture Vaccines Market Analysis - Size, Share, and Forecast Outlook for 2025 to 2035
Recombinant Vaccines Market Analysis – Trends & Future Outlook 2018-2028
Pneumococcal Vaccines Market Size and Share Forecast Outlook 2025 to 2035
Anti-Infective Vaccines Market Growth – Trends & Forecast 2025 to 2035
The Companion Animal Vaccines Market is segmented by product, indication and end user from 2025 to 2035
Chile Aquaculture Vaccines Market Insights – Size, Demand & Forecast 2025-2035
China Aquaculture Vaccines Market Outlook – Growth & Forecast 2025-2035
Seasonal Influenza Vaccines Therapeutics Market Size and Share Forecast Outlook 2025 to 2035
Norway Aquaculture Vaccines Market Insights – Size, Demand & Growth 2025-2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA