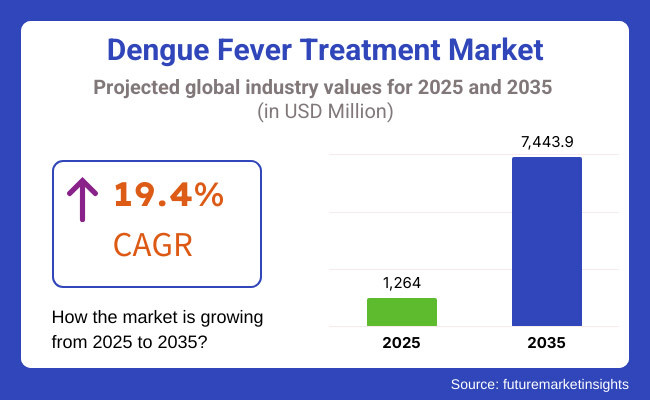
The market is projected to reach USD 1,264 Million in 2025 and is expected to grow to USD 7,443.9 Million by 2035, registering a CAGR of 19.4% over the forecast period. The growth of vector control programs, adoption of AI-powered disease surveillance systems, and introduction of monoclonal antibody treatments are shaping the industry's future.
Additionally, rising healthcare infrastructure investments in dengue-endemic regions and expansion of clinical trials for novel therapeutics are driving new opportunities. Driven by a growing global incidence of dengue infections and surging government initiatives for disease control, the Dengue Fever Treatment Market is expected to grow impressively between 2025 to 2035.
The Dengue Virus causes dengue fever. Aedes is the primary transmitter of this disease, which continues to haunt public health work especially in tropical and subtropical regions where there are highly favorable environmental conditions which encourage mosquito breeding.
All over the world, governments plus medical care organizations are increasing their efforts to ease the load of this disease, using anything from strategies for vector control to technical innovation - industrial surveillance networks and public awareness campaigns.
In addition to this, the burgeoning of dengue vaccination programs will also take an active part in reducing patient illness severity and hospitalization rate. Combined with the increasing research and development work done in the pharmaceutical industry, these contributions are making the market ever larger.
In addition, treatments that are becoming available in the international market for dengue fever fever treatment include developments directed against norovirus, including a few new antivirals and one vaccine. Novel antiviral therapies that aim directly at the virus itself are still picking up steam. But patients' expectations are high and many drug candidates are already undergoing testing in phase I clinical trials.
Multiple rapid diagnostic tests and point-of-care testing solutions are now available, offering early diagnosis and timely management with advantages to human life itself. For instance, this means more good years in children's lives as well that people no longer fear catching an illness because they do know that medicine can help get them back on their feet.
The treatment is currently mainly for the support type, consisting of fluids such as blood serum and intravenous drips Hand, and pain control Titan, and for every serious case medical treatment in hospital remains necessary. This is particularly valid in areas such as South America where diseases keep recurring.
The enthusiasm and capital from pharmaceutical companies, biotechnology firms, as well global health organizations, are gradually introducing new ways for treating the disease. Consequently, it is predicted that this market will continue to develop strongly, due to both technological advances and collaborative efforts between public and private parties.
Due to the increasing research into antiviral drug development, a rise in dengue cases as a result of climate change and the inoculation programs that have expanded, North America is expected to hold a substantial share of the market for dengue fever treatment.
The USA and Mexico hold the lead for two reasons, one country is increasing its funding to carry out vector control measures; another is perfecting its AI-based mosquito-tracking systems. In addition, diagnostic technologies for dengue are so advanced in both countries.
The USA FDA as well as agencies such as the Centers for Disease Control and Prevention are behind research into monoclonal antibody therapies for fighting dengue fever a long with dengue-specific antivirals. It is the expansion in clinical trials of new generation dengue vaccines and rapid diagnostic kits that is moving forward market growth.
The Dengue Fever Treatment Market has a fair share in Europe, The countries of France, Spain, Britain Italy and others all know only too well that it their responsibility to bear for surveillance of disease prevention. The European Center for Disease Prevention and Control has launched a campaign to prevent infection from dengue. It is also investing in innovative detection technology.
The upswing of infectious disease has led to an upturn for dengue testing, telemedicine scripts and even AI-controlled vector surveillance systems. Moderna, Pfizer, AstraZeneca Taj Pharmaceuticals and Indian universities are all now licensed to produce dengue-targeting monoclonal antibodies.
High dengue incidence and investment in infrastructure in the health care sector will give the Asia-Pacific region a better CAGR. Furthermore, existing government-powered mosquito control programs expand even further with immunizations for everyone. India, Thailand, Indonesia, the Philippines, Vietnam are all countries that are forward in terms of endemic dengue disease, rapid testing options and research and development in antiviral drugs.
India's National Vector Borne Disease Control Programme (NVBDCP) and Thailand's Dengue Strategic Plan are pushing mass dengue monitoring, insecticide-treated bed net distribution and patient management solutions.
The Dengvaxia vaccine has generated positive word-of-mouth and increased business for AI-powered mosquito hotspot tracking technology; far-flung locales were mentioned in combination with this company as where they intended to sell their already established system. There is also telemedicine intensified dengue patient care, and all of these factors combined create additional market demand.
Challenges
Lack of Targeted Antiviral Treatments and Vaccine Accessibility
The dominant challenge in the Dengue Fever Treatment Market is that there are no specificity antiviral drugs, but instead current treatments focus simply on relieving symptoms (typically fever reduction, fluid replacement and pain management).
High cost barriers, regulatory challenges and cold-chain distribution issues keep dengue vaccines out of many high-incidence areas in the low-income regions with which they are most clearly linked, thus also limiting market territory available for extension.
The complexity of the dengue virus serotypes (DENV-1, DENV-2, DENV-3, DENV-4) and the possibility that antibody-dependent enhancement (ADE) may occur in vaccines present both scientific and safety concerns for global immunization strategies.
Opportunities
AI-Based Vector Control, Monoclonal Antibody Therapies, and Next-Generation Vaccines
Despite challenges, the Dengue Fever Treatment Market presents significant growth opportunities. Artificial intelligence systems that track mosquitoes and predict potential outbreaks of the virus, along with programs to genetically sterilize the mosquito population have greatly improved our efforts at controlling this vector.
Various measures are being adopted to enhance treatment. Modern biopharmaceuticals, or monolithic drugs, such as Janssen’s JNJ-1802 and Takeda’s TAK-003 vaccine, have developed targeted therapies with much greater efficacy against multiple dengue serotypes. Moreover, ongoing clinical trials for all serotypes at once pan-dengue vaccines are likely to extend their use from only the most seriously affected regions to endemic areas generally.
Rapid diagnostic tools, using technology that is both portable and artificial intelligence powered as well as rapid, are helping to spot the disease early. Companies and organizations are seeking novel solutions for home-based supportive care, and a number of digital health monitoring apps have been developed.
Wireless dengue consultations are also growing in popularity which means that many patients can take advantage of phone or video conference instead commuting Pig home and back from hospital clinics.
The period from 2020 to 2024 saw a boom in the dengue fever treatment market. World dengue cases have in recent years been rising; climate change has brought about more mosquitoes; and greater investment in antiviral research development all facilitated this growth. Dengue, a mosquito-borne viral disease, is mostly prevalent in tropical and subtropical areas.
Asian-Pacific countries, Latin America and Africa have normally re-time violent outbreaks every 2-3 years Price differences for antivirals accounted for more than 30% of price hesitation. The market concentrated on symptomatic therapies such as fluid replacement therapy, pain relief medicines (paracetamol) and supportive hospital care.
Between 2025 and 2035, the dengue fever treatment market will be different. There will be the rise of AI-assisted antiviral drug discovery, gene editing mosquito control strategy, and next-generation vaccine platforms. CRISPR-based vector control will transform mosquito population suppression methods, lowering transmission rates. AI-powered diagnostic tools will offer real-time, at-home dengue detection, thus facilitating early intervention and outbreak containment.
Market Shifts: A Comparative Analysis (2020 to 2024 vs. 2025 to 2035)
| Market Shift | 2020 to 2024 |
|---|---|
| Regulatory Landscape | WHO and FDA approvals for Dengvaxia and Qdenga vaccines, diagnostic kit validation, and global mosquito control programs. |
| Technological Advancements | Growth in PCR-based dengue diagnostics, vaccine research, and AI-powered outbreak prediction models. |
| Industry Applications | Used in hospital care, government vaccination programs, and public health initiatives. |
| Adoption of Smart Equipment | Integration of rapid test kits, AI-based epidemiological tracking, and cloud-based patient data analytics. |
| Sustainability & Cost Efficiency | Shift toward affordable dengue vaccines, sustainable mosquito control methods, and AI-driven resource allocation. |
| Data Analytics & Predictive Modeling | Use of machine learning for outbreak prediction, AI-assisted drug repurposing, and mobile-based fever tracking. |
| Production & Supply Chain Dynamics | Challenges in high vaccine costs, complex distribution logistics, and regional healthcare disparities. |
| Market Growth Drivers | Growth fueled by rising dengue cases, climate change effects, and expanding vector control efforts. |
| Market Shift | 2025 to 2035 |
|---|---|
| Regulatory Landscape | Blockchain-backed vaccine tracking, AI-driven vector surveillance, and CRISPR-based mosquito gene editing regulations. |
| Technological Advancements | Next-gen antiviral therapies, real-time AI diagnostics, and nanotechnology-enhanced dengue treatment. |
| Industry Applications | Expanded into AI-driven personalized dengue treatment, decentralized vaccine production, and smart mosquito monitoring networks. |
| Adoption of Smart Equipment | Wearable dengue detection biosensors, real-time AI-driven outbreak mapping, and blockchain-enabled global response coordination. |
| Sustainability & Cost Efficiency | Carbon-neutral vaccine manufacturing, AI-optimized epidemic response logistics, and decentralized vector control strategies. |
| Data Analytics & Predictive Modeling | Quantum-enhanced viral evolution modeling, AI-driven precision antiviral therapy, and blockchain-backed pandemic prevention. |
| Production & Supply Chain Dynamics | Decentralized vaccine hubs, AI-driven supply chain automation, and blockchain-powered transparent vaccine distribution. |
| Market Growth Drivers | Future expansion driven by AI-enhanced public health strategies, genetic mosquito control, and next-gen antiviral developments. |
In United States, the Dengue Fever Treatment Market is now Earthmoving due to the ever wider spread of mosquito-born diseases because of a warming climate, ever larger investments in research projects for anti-viral therapy and progress with the development of vaccines against dengue fever.
Centers for Disease Control and Prevention (CDC) and The National Institutes of Health (NIH) actively support dengue surveillance, testing vaccine prodromes as well as new antiviral drug research.
The adoption of monoclonal antibody therapies, improved vector control strategies, and AI-driven predictive modeling for outbreak management is shaping market trends. Additionally, rising travel-related infections and increasing healthcare infrastructure for tropical diseases are further boosting market demand.
| Country | CAGR (2025 to 2035) |
|---|---|
| USA | 19.7% |
With an increasing global travel exposure and the target of tropical disease research, the Dengue Fever Treatment Market in the United Kingdom is expanding. Strong government initiatives for infectious disease control also contribute to this trend. The UK Health Security Agency (UKHSA) and the Wellcome Trust are supporting clinical research into dengue antivirals, vaccine development, and rapid diagnostics.
Leveraging partnerships between pharmaceutical firms and research institutions allows for progress in dengue immunotherapy and the development of new drug forms. The government gives more money to fight vector surveillance and outbreak readiness, which also contributes additional market expansion.
| Country | CAGR (2025 to 2035) |
|---|---|
| UK | 18.9% |
With the rise of climate-related diseases, dengue fever outbreaks have been on the increase in Europe. Southern Europe is facing more and more cases of dengue fever, and the European Centre for Disease Prevention and Control (ECDC) as well as its Horizon Europe Program is giving financial support towards turning the discoveries about darged viruses into actual products.
Dengue therapeutics clinical trials are being conducted in Germany, France and Spain, and vaccine distribution programs such as for distributing Dengvaxia are already underway. There are also rapid diagnostic tests developing in these countries. Another major expansion consists of the global disease surveillance networks, as well as AI-based epidemiology modeling that helps improve outbreak preparedness.
| Country | CAGR (2025 to 2035) |
|---|---|
| European Union (EU) | 19.4% |
The Dengue Fever Treatment Market in Japan is developing because it contains increasing state efforts to prevent vector diseases, rehabilitation efforts (especially in the dengue fever treatment field), and more research into tropical medicine. Japanese Ministry of Health, Labor and Welfare (MHLW) is actively funding advanced dengue diagnostic tests as well as drug research.
Japanese pharmaceutical companies are taking the lead in dengue vaccine research, AI-driven outbreak prediction models and molecular dengue detection kits. Furthermore, the increasing integration of nanotechnology into antiviral drug delivery systems is improving treatment efficacy.
| Country | CAGR (2025 to 2035) |
|---|---|
| Japan | 19.6% |
South Korea's Dengue Fever Treatment Market is thriving. This is the outcome of increased research collaborations on infectious disease; a tendency toward dengue fever prevalence changes due to climate shifts; and input from governments around the world that have prepared big financial packages for epidemic control. In South Korea, the Ministry of Health and Welfare is investing hugely in advanced vector control measures and also innovative vaccines.
South Korean biotech companies are working on next-generation dengue vaccines, AI-assisted disease prediction platforms, and targeted antiviral therapies. Moreover, the development of mobile health monitoring apps to track dengue symptoms is not only bringing improved patient care but is also every efficiently collecting data on outbreaks.
| Country | CAGR (2025 to 2035) |
|---|---|
| South Korea | 19.8% |
The Dengue Fever Treatment Market is growing because dengue outbreaks are on the rise, mosquito-borne illness is increasing in prevalence, there is significant progress in antiviral drug development and vaccination efforts. Among the various types of treatment, drugs in the market take up much of it offering symptomatic relief as well as long-term immunity against dengue viruses.
Drugs Lead Market Demand with Supportive Treatment and Symptom Management
At present, the main treatment for dengue fever is antiviral treatment, as there is no cure specifically for this disease as yet. These drugs are aimed at giving symptomatic relief, lowing speeds of fever progression and controlling the pain; serious complications such as hemorrhagic fever or dengue shock syndrome are thus averted.
The prevalent use of acetaminophen (paracetamol) as the first choice in many countries for reducing fever, together with fluid replacement therapies, platelet transfusions and supportive care, has pushed this market further on. Also in the field of research for antivirals targeting dengue viral replications there is movement. In the next few years this work is likely to bring more options for treating the disease.
Non-steroidal anti-inflammatory drugs (NSAID) are widely used despite the fact that virus-specific drugs are available. A big challenge for people with dengue is bleeding complications from taking ibuprofen and other similar drugs. However, research into a dengue antiviral, combination drug therapies, and AI-assisted targeting of drug repurposing are all expected to improve treatment efficacy as well as reduce mortality rates.
Vaccines Gain Traction as Preventive Strategies Expand in Endemic Regions
Dengue vaccines have become an essential means to achieve herd immunity in the long term, control outbreaks and contain disease in high-risk zones. Tetravalent vaccines against all four dengue virus serotypes are increasing vaccine effectiveness and helping drive market demand.
Huge vaccination campaigns, especially in dengue-endemic countries are currently being pushed by Sanofi Pasteur's Dengvaxia and Takeda Pharmaceuticals' Qdenga receiving approval from multiple regions. In addition, future dengue vaccine products with better efficacy and broader serotype spectrum protection are expected to increase the availability of vaccines worldwide.
There are still many obstacles, such as the fact that seronegative individuals may receive limited protection from the vaccine and that the process may carry risks of antibody-dependent enhancement (ADE). However, the use of live-attenuated and mRNA-based vaccine technologies combined with WHO-supported immunization initiatives should increase vaccine effectiveness and market growth prospects.
The market for dengue fever drugs is dominated by preferences of routes of administration, where oral and parenteral drugs are the most popular because they are convenient, effective, and used both in managing symptoms and preventing disease.
Oral Administration Leads Market Demand for Convenience and Outpatient Treatment
Oral drugs are still the most commonly used treatment of dengue fever due to their exceptional ease of administration, cost efficiency and wide availability. These include fever reducers, pain killers and hydration water by way of dengue fever symptoms and preventing dehydration.
While oral formulations of antiviral drug candidates become increasingly available and AI-assisted drug discovery efforts repurpose existing oral medications for dengue, the market scale continues to grow. Oral rehydration therapy (ORT) and electrolyte supplements are crucial in preventing severe complications as well as reduce hospitalization rate.
Despite widespread adoption, there are still challenges such as delayed effect in severe dengue and lower compliance rates among paediatric and elderly patients; but long-acting oral antivirals as well as oral drug combinations which greatly reduce the frequency of dosing over time are expected to improve future treatments.
Parenteral Administration Gains Popularity for Severe Dengue Cases and Hospitalized Patients
Parenteral (intramuscular/intravenous) therapy, i.e., IV fluids, platelet transfusion, and vaccination injection, is needed in case of serious cases of dengue that are admitted to hospital and require preventive immunization procedures. The therapy is used in intensive care units for treating the complications of plasma leakage, haemorrhagic fever, and shock syndrome.
This is further accelerating market demand, mainly because in areas where annual epidemics take place such as Southeast Asia and the Pacific, the rate of inoculation with parenterally administered Dengue fever vaccines has increased recently. Meanwhile, future therapies for severe or life-threatening dengue infections such as IV-administered monoclonal antibodies and plasma-derived products are currently in development.
Despite its importance, barriers to broad utilization remain. They include high treatment costs, the need for trained personnel, and difficulty in getting to remote rural districts. However, innovative approaches to long-lasting IV immunotherapies, subcutaneous dengue vaccine formulations, and hospital-based rapid dengue management protocols are anticipated to facilitate access and improve patient outcomes.
The treatment market for Dengue Fever is expanding due to the increasing global prevalence of dengue infections, expanding research into antiviral medications, and developing supportive therapies and vaccines. It is stimulated by expanding outbreaks in tropical and subtropical regions, government-supported control programs for dengue, and increased diagnostic capabilities.
The focus of companies lies in antiviral medicines, vaccine creation, supportive care treatments, and vector control measures for improving patient results, disease treatment, and prevention. The market consists of prominent pharmaceutical companies, biotechnology corporations, and manufacturers of diagnostic tests, all driving innovations in monoclonal antibody treatments, quick diagnostic kits, and dengue vaccines.
Market Share Analysis by Company
| Company Name | Estimated Market Share (%) |
|---|---|
| Sanofi S.A. | 18-22% |
| Takeda Pharmaceutical Company | 12-16% |
| Johnson & Johnson (Janssen Pharmaceuticals) | 10-14% |
| GlaxoSmithKline plc (GSK) | 8-12% |
| Merck & Co., Inc. | 6-10% |
| Other Companies (combined) | 30-40% |
| Company Name | Key Offerings/Activities |
|---|---|
| Sanofi S.A. | Developed Dengvaxia®, the first approved dengue vaccine, for prevention in endemic regions. |
| Takeda Pharmaceutical Company | Launched Qdenga® (TAK-003), a tetravalent dengue vaccine with broader protection. |
| Johnson & Johnson (Janssen Pharmaceuticals) | Engaged in antiviral drug research and vaccine development for dengue treatment. |
| GlaxoSmithKline plc (GSK) | Focuses on vector control solutions and supportive care medications for dengue patients. |
| Merck & Co., Inc. | Conducts clinical trials on monoclonal antibodies for dengue virus neutralization. |
Key Company Insights
Sanofi S.A. (18-22%)
Sanofi is the market leader in dengue prevention, with Dengvaxia® being the first WHO-approved dengue vaccine, widely used in endemic regions.
Takeda Pharmaceutical Company (12-16%)
Takeda’s Qdenga® vaccine (TAK-003) offers broader protection against all four dengue serotypes, positioning it as a strong competitor to Dengvaxia®.
Johnson & Johnson (Janssen Pharmaceuticals) (10-14%)
Janssen is involved in antiviral drug discovery and clinical research, aiming to develop effective therapeutic options for dengue fever management.
GlaxoSmithKline plc (GSK) (8-12%)
GSK focuses on vector control and disease management solutions, supporting dengue prevention alongside vaccine development.
Merck & Co., Inc. (6-10%)
Merck is conducting clinical research on monoclonal antibody treatments, aiming for therapeutic solutions to neutralize the dengue virus.
Other Key Players (30-40% Combined)
Several biopharma firms, diagnostics companies, and public health organizations contribute to dengue treatment advancements, vaccine development, and rapid testing solutions. These include:
The overall market size for the Dengue Fever Treatment Market was USD 1,264 Million in 2025.
The Dengue Fever Treatment Market is expected to reach USD 7,443.9 Million in 2035.
Rising global incidence of dengue fever, increasing government initiatives for vector control, and advancements in antiviral drug research and vaccine development will drive market growth.
India, Brazil, Indonesia, Thailand, and the Philippines are key contributors.
Drugs and Vaccines are expected to lead in the Dengue Fever Treatment Market.
Table 1: Global Value (US$ Million) Forecast by Region, 2018 to 2033
Table 2: Global Value (US$ Million) Forecast by Type, 2018 to 2033
Table 3: Global Value (US$ Million) Forecast by Route of Administration, 2018 to 2033
Table 4: Global Value (US$ Million) Forecast by End-user, 2018 to 2033
Table 5: North America Value (US$ Million) Forecast by Country, 2018 to 2033
Table 6: North America Value (US$ Million) Forecast by Type, 2018 to 2033
Table 7: North America Value (US$ Million) Forecast by Route of Administration, 2018 to 2033
Table 8: North America Value (US$ Million) Forecast by End-user, 2018 to 2033
Table 9: Latin America Value (US$ Million) Forecast by Country, 2018 to 2033
Table 10: Latin America Value (US$ Million) Forecast by Type, 2018 to 2033
Table 11: Latin America Value (US$ Million) Forecast by Route of Administration, 2018 to 2033
Table 12: Latin America Value (US$ Million) Forecast by End-user, 2018 to 2033
Table 13: Europe Value (US$ Million) Forecast by Country, 2018 to 2033
Table 14: Europe Value (US$ Million) Forecast by Type, 2018 to 2033
Table 15: Europe Value (US$ Million) Forecast by Route of Administration, 2018 to 2033
Table 16: Europe Value (US$ Million) Forecast by End-user, 2018 to 2033
Table 17: Asia Pacific Value (US$ Million) Forecast by Country, 2018 to 2033
Table 18: Asia Pacific Value (US$ Million) Forecast by Type, 2018 to 2033
Table 19: Asia Pacific Value (US$ Million) Forecast by Route of Administration, 2018 to 2033
Table 20: Asia Pacific Value (US$ Million) Forecast by End-user, 2018 to 2033
Table 21: MEA Value (US$ Million) Forecast by Country, 2018 to 2033
Table 22: MEA Value (US$ Million) Forecast by Type, 2018 to 2033
Table 23: MEA Value (US$ Million) Forecast by Route of Administration, 2018 to 2033
Table 24: MEA Value (US$ Million) Forecast by End-user, 2018 to 2033
Figure 1: Global Value (US$ Million) by Type, 2023 to 2033
Figure 2: Global Value (US$ Million) by Route of Administration, 2023 to 2033
Figure 3: Global Value (US$ Million) by End-user, 2023 to 2033
Figure 4: Global Value (US$ Million) by Region, 2023 to 2033
Figure 5: Global Value (US$ Million) Analysis by Region, 2018 to 2033
Figure 6: Global Value Share (%) and BPS Analysis by Region, 2023 to 2033
Figure 7: Global Y-o-Y Growth (%) Projections by Region, 2023 to 2033
Figure 8: Global Value (US$ Million) Analysis by Type, 2018 to 2033
Figure 9: Global Value Share (%) and BPS Analysis by Type, 2023 to 2033
Figure 10: Global Y-o-Y Growth (%) Projections by Type, 2023 to 2033
Figure 11: Global Value (US$ Million) Analysis by Route of Administration, 2018 to 2033
Figure 12: Global Value Share (%) and BPS Analysis by Route of Administration, 2023 to 2033
Figure 13: Global Y-o-Y Growth (%) Projections by Route of Administration, 2023 to 2033
Figure 14: Global Value (US$ Million) Analysis by End-user, 2018 to 2033
Figure 15: Global Value Share (%) and BPS Analysis by End-user, 2023 to 2033
Figure 16: Global Y-o-Y Growth (%) Projections by End-user, 2023 to 2033
Figure 17: Global Attractiveness by Type, 2023 to 2033
Figure 18: Global Attractiveness by Route of Administration, 2023 to 2033
Figure 19: Global Attractiveness by End-user, 2023 to 2033
Figure 20: Global Attractiveness by Region, 2023 to 2033
Figure 21: North America Value (US$ Million) by Type, 2023 to 2033
Figure 22: North America Value (US$ Million) by Route of Administration, 2023 to 2033
Figure 23: North America Value (US$ Million) by End-user, 2023 to 2033
Figure 24: North America Value (US$ Million) by Country, 2023 to 2033
Figure 25: North America Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 26: North America Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 27: North America Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 28: North America Value (US$ Million) Analysis by Type, 2018 to 2033
Figure 29: North America Value Share (%) and BPS Analysis by Type, 2023 to 2033
Figure 30: North America Y-o-Y Growth (%) Projections by Type, 2023 to 2033
Figure 31: North America Value (US$ Million) Analysis by Route of Administration, 2018 to 2033
Figure 32: North America Value Share (%) and BPS Analysis by Route of Administration, 2023 to 2033
Figure 33: North America Y-o-Y Growth (%) Projections by Route of Administration, 2023 to 2033
Figure 34: North America Value (US$ Million) Analysis by End-user, 2018 to 2033
Figure 35: North America Value Share (%) and BPS Analysis by End-user, 2023 to 2033
Figure 36: North America Y-o-Y Growth (%) Projections by End-user, 2023 to 2033
Figure 37: North America Attractiveness by Type, 2023 to 2033
Figure 38: North America Attractiveness by Route of Administration, 2023 to 2033
Figure 39: North America Attractiveness by End-user, 2023 to 2033
Figure 40: North America Attractiveness by Country, 2023 to 2033
Figure 41: Latin America Value (US$ Million) by Type, 2023 to 2033
Figure 42: Latin America Value (US$ Million) by Route of Administration, 2023 to 2033
Figure 43: Latin America Value (US$ Million) by End-user, 2023 to 2033
Figure 44: Latin America Value (US$ Million) by Country, 2023 to 2033
Figure 45: Latin America Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 46: Latin America Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 47: Latin America Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 48: Latin America Value (US$ Million) Analysis by Type, 2018 to 2033
Figure 49: Latin America Value Share (%) and BPS Analysis by Type, 2023 to 2033
Figure 50: Latin America Y-o-Y Growth (%) Projections by Type, 2023 to 2033
Figure 51: Latin America Value (US$ Million) Analysis by Route of Administration, 2018 to 2033
Figure 52: Latin America Value Share (%) and BPS Analysis by Route of Administration, 2023 to 2033
Figure 53: Latin America Y-o-Y Growth (%) Projections by Route of Administration, 2023 to 2033
Figure 54: Latin America Value (US$ Million) Analysis by End-user, 2018 to 2033
Figure 55: Latin America Value Share (%) and BPS Analysis by End-user, 2023 to 2033
Figure 56: Latin America Y-o-Y Growth (%) Projections by End-user, 2023 to 2033
Figure 57: Latin America Attractiveness by Type, 2023 to 2033
Figure 58: Latin America Attractiveness by Route of Administration, 2023 to 2033
Figure 59: Latin America Attractiveness by End-user, 2023 to 2033
Figure 60: Latin America Attractiveness by Country, 2023 to 2033
Figure 61: Europe Value (US$ Million) by Type, 2023 to 2033
Figure 62: Europe Value (US$ Million) by Route of Administration, 2023 to 2033
Figure 63: Europe Value (US$ Million) by End-user, 2023 to 2033
Figure 64: Europe Value (US$ Million) by Country, 2023 to 2033
Figure 65: Europe Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 66: Europe Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 67: Europe Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 68: Europe Value (US$ Million) Analysis by Type, 2018 to 2033
Figure 69: Europe Value Share (%) and BPS Analysis by Type, 2023 to 2033
Figure 70: Europe Y-o-Y Growth (%) Projections by Type, 2023 to 2033
Figure 71: Europe Value (US$ Million) Analysis by Route of Administration, 2018 to 2033
Figure 72: Europe Value Share (%) and BPS Analysis by Route of Administration, 2023 to 2033
Figure 73: Europe Y-o-Y Growth (%) Projections by Route of Administration, 2023 to 2033
Figure 74: Europe Value (US$ Million) Analysis by End-user, 2018 to 2033
Figure 75: Europe Value Share (%) and BPS Analysis by End-user, 2023 to 2033
Figure 76: Europe Y-o-Y Growth (%) Projections by End-user, 2023 to 2033
Figure 77: Europe Attractiveness by Type, 2023 to 2033
Figure 78: Europe Attractiveness by Route of Administration, 2023 to 2033
Figure 79: Europe Attractiveness by End-user, 2023 to 2033
Figure 80: Europe Attractiveness by Country, 2023 to 2033
Figure 81: Asia Pacific Value (US$ Million) by Type, 2023 to 2033
Figure 82: Asia Pacific Value (US$ Million) by Route of Administration, 2023 to 2033
Figure 83: Asia Pacific Value (US$ Million) by End-user, 2023 to 2033
Figure 84: Asia Pacific Value (US$ Million) by Country, 2023 to 2033
Figure 85: Asia Pacific Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 86: Asia Pacific Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 87: Asia Pacific Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 88: Asia Pacific Value (US$ Million) Analysis by Type, 2018 to 2033
Figure 89: Asia Pacific Value Share (%) and BPS Analysis by Type, 2023 to 2033
Figure 90: Asia Pacific Y-o-Y Growth (%) Projections by Type, 2023 to 2033
Figure 91: Asia Pacific Value (US$ Million) Analysis by Route of Administration, 2018 to 2033
Figure 92: Asia Pacific Value Share (%) and BPS Analysis by Route of Administration, 2023 to 2033
Figure 93: Asia Pacific Y-o-Y Growth (%) Projections by Route of Administration, 2023 to 2033
Figure 94: Asia Pacific Value (US$ Million) Analysis by End-user, 2018 to 2033
Figure 95: Asia Pacific Value Share (%) and BPS Analysis by End-user, 2023 to 2033
Figure 96: Asia Pacific Y-o-Y Growth (%) Projections by End-user, 2023 to 2033
Figure 97: Asia Pacific Attractiveness by Type, 2023 to 2033
Figure 98: Asia Pacific Attractiveness by Route of Administration, 2023 to 2033
Figure 99: Asia Pacific Attractiveness by End-user, 2023 to 2033
Figure 100: Asia Pacific Attractiveness by Country, 2023 to 2033
Figure 101: MEA Value (US$ Million) by Type, 2023 to 2033
Figure 102: MEA Value (US$ Million) by Route of Administration, 2023 to 2033
Figure 103: MEA Value (US$ Million) by End-user, 2023 to 2033
Figure 104: MEA Value (US$ Million) by Country, 2023 to 2033
Figure 105: MEA Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 106: MEA Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 107: MEA Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 108: MEA Value (US$ Million) Analysis by Type, 2018 to 2033
Figure 109: MEA Value Share (%) and BPS Analysis by Type, 2023 to 2033
Figure 110: MEA Y-o-Y Growth (%) Projections by Type, 2023 to 2033
Figure 111: MEA Value (US$ Million) Analysis by Route of Administration, 2018 to 2033
Figure 112: MEA Value Share (%) and BPS Analysis by Route of Administration, 2023 to 2033
Figure 113: MEA Y-o-Y Growth (%) Projections by Route of Administration, 2023 to 2033
Figure 114: MEA Value (US$ Million) Analysis by End-user, 2018 to 2033
Figure 115: MEA Value Share (%) and BPS Analysis by End-user, 2023 to 2033
Figure 116: MEA Y-o-Y Growth (%) Projections by End-user, 2023 to 2033
Figure 117: MEA Attractiveness by Type, 2023 to 2033
Figure 118: MEA Attractiveness by Route of Administration, 2023 to 2033
Figure 119: MEA Attractiveness by End-user, 2023 to 2033
Figure 120: MEA Attractiveness by Country, 2023 to 2033






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Dengue Vaccines Analysis by Product Type by Product, By Age Group and by Distribution Channel through 2035
Infant Fever Stickers Market Size and Share Forecast Outlook 2025 to 2035
Yellow Fever Treatment Market
Brazilian Hemorrhagic Fever (BzHF) Treatment Market
Treatment-Resistant Hypertension Management Market Size and Share Forecast Outlook 2025 to 2035
Treatment-Resistant Depression Treatment Market Size and Share Forecast Outlook 2025 to 2035
Treatment Pumps Market Insights Growth & Demand Forecast 2025 to 2035
Pretreatment Coatings Market Size and Share Forecast Outlook 2025 to 2035
Air Treatment Ozone Generator Market Size and Share Forecast Outlook 2025 to 2035
CNS Treatment and Therapy Market Insights - Trends & Growth Forecast 2025 to 2035
Seed Treatment Materials Market Size and Share Forecast Outlook 2025 to 2035
Acne Treatment Solutions Market Size and Share Forecast Outlook 2025 to 2035
Scar Treatment Market Overview - Growth & Demand Forecast 2025 to 2035
Soil Treatment Chemicals Market
Water Treatment System Market Size and Share Forecast Outlook 2025 to 2035
Water Treatment Chemical Market Size and Share Forecast Outlook 2025 to 2035
Algae Treatment Chemical Market Forecast and Outlook 2025 to 2035
Water Treatment Market Size and Share Forecast Outlook 2025 to 2035
Water Treatment Ozone Generator Market Size and Share Forecast Outlook 2025 to 2035
Water Treatment Equipment Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA