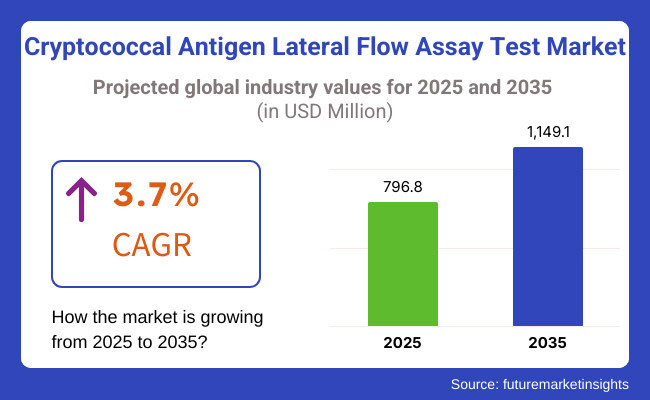
In the coming years the cryptococcal antigen lateral flow assay test products market is expected to reach USD 796.8 million by 2025 and is expected to steadily grow at a CAGR of 3.7% to reach USD 1,149.1 million by 2035. In 2024, cryptococcal antigen lateral flow assay test have generated roughly USD 768.1 million in revenues.
The CrAg LFA is a point-of-care test for the detection of cryptococcal antigens in blood or cerebrospinal fluid for diagnosis of Cryptococcus infection. This infection is usually detected in patients who are immunocompromised. The CrAg LFA is highly beneficial as an easy, quick, and precise means of diagnosing cryptococcal meningitis, which can be deadly if not treated.
There are a number of advantages for using this test: One, patients get results within an hour of taking the test, and there is no possibility of any delay in treatment, which can be days before being seen with conventional culture. It is fairly inexpensive, doesn't require much equipment, making it perform well with the low resource setup.
Since it is increasing in the burden of HIV/AIDS and fungal infection per head of the global population, it has found acceptance early diagnosis and treatment purposes in clinical practice worldwide, improving patients' outcomes.
Over the years of 2020 and 2024, a series of historical influences contributed to the uptake of the cryptococcal antigen lateral flow assay-crAg lateral flow assay. The COVID-19 pandemic was the main focus, with all nations turning attention toward fast, cheap warehouses to facilitate diagnostics. This triggered funding in the field of diagnostics technology and further highlighted the value of tests such as CrAg LFA that provide rapid results requiring less infrastructure.
One clear reason would be rapid and cheap diagnostics, especially, on the local scenario of the continued global burden of HIV/ AIDS still in sub-Saharan Africa, a place heavily struck down with deaths by the cryptococcal meningitis, which largely poses a very serious indication for people to recognize that the need for rapid and cheap diagnostics is extensive. Over this time, many global health organizations, among them WHO, have called for increased access to CrAg LFA in resource-limited settings, which is would reduce mortality.
On top of that, improvements in global health intervention, intensified sensitization, and better funding in diagnostic equipment have all resulted in the extended use of CrAg LFA as a valuable tool in combating fungal infection.
Explore FMI!
Book a free demo
The rising awareness of cryptococcal infections, especially in immunocompromised patients like those infected with HIV/AIDS, has significantly contributed to the growth of the market. Moreover, the rapidity and cost-effectiveness added to the clinical appeal of CrAg LFA.
This compares positively with classical methods, including culture tests, which are tedious and require sophisticated laboratory setups; the CrAg LFA provides answers within hours to facilitate speedy treatment decisions that are life-saving for an acute pathogen like cryptococcal meningitis.
Furthermore, the robust health sector of North America and commitment to improving equity in the health system have been conducive to the wide-scale application of CrAg LFA. It has been made even more accessible as it can solve problems in various health setups, including point-of-care settings in periphery areas or outpatient clinics.
Consequently, an increasing number of partnerships among health-related organizations, public health institutions, and research facilities have led to more investment in fungal diagnostics. This increased priority given to testing has inflated interest in the test itself, particularly with regard to therapy for fungal infections, which have historically been underdiagnosed in the region.
The patient-centered care devolution that has been taking place in the European healthcare system has aided CrAG LFA test market to capture a significant market share in the European countries. User-friendly, low-level training, and easy-to-use diagnostic tools such as CrAg LFA are definitely worth it in urban hospitals and in more rural or underserved areas.
The body's strong intention to fight against antimicrobial resistance within Europe has fostered investment in creating diagnostic technologies that could prevent unnecessary treatments. CrAg LFA directs therapy and cuts down on antifungal misuse cases through accurate diagnosis of cryptococcal infection.
Ultimately, these have improved access to the test through government grants and advocacy for better access to diagnostics, especially in countries where the HIV burden is higher, linked with joint work between research and public health institutions in Europe.
Accruements continue in the Asia Pacific zone, attributing a gamut of reasons for purchasing of sales for the Cryptococcal Antigen Lateral Flow Assay (CrAg LFA) test. Countries like India and Thailand, where HIV infection rates are relatively high, have reported increased demand for rapid tests such as CrAg LFA to improve diagnosis of cryptococcal meningitis and enhance patient outcomes.
Moreover, improving healthcare infrastructure and emphasizing disease detection in rural and under-privileged communities favor taking up the test. Simple and speedy, low-cost nature of CrAg LFA test is ideal for low-resource locations where infrastructure and laboratories for sophisticated testing facilities are hard to access. This is crucial in complementing the region's pursuit for closing the inequities in health sectors and increasing access to diagnostics.
Last, world health agencies, including the World Health Organization, have been more than catalysts for the advocacy of CrAg LFA use by supporting efforts to control fungal infections in the Asia Pacific region. Increased awareness of fungal diseases and investments in research have also driven sales of tests in the entire region.
Challenges
Accuracy and Sensitivity Issues Related to the Cryptococcal Antigen Lateral Flow Assay test hinders its Adoption in the Market
One of the tests CrAg LFA faces is reliability and sensitivity when dealing with certain populations of patients. While very good at detecting cryptococcal meningitis in the immunocompromised patient, CrAg LFA might lose some sensitivity in patients with low-fungal burden or early infection. The result is a fair number of false negatives which subsequently delay or complicate treatment.
Moreover, differences in performance of tests from one setting to another (for example, rural or less-resourced places) can undermine their reliability. Though these are generally reliable tests, there is a continued need for validation and quality control, alongside training of health professionals, so that incidences of diagnostic errors are cut down and patient outcomes improved as they proliferate in their application and use over many settings.
Opportunities
Emphasis of Manufacturers on Collaboration with Global Health Program Organization create New Business Opportunities for the Market Players
Emphasis on introduction of this test kits into global health programs against fungal diseases pose significant opportunities for this market. An increase in public awareness of fungal diseases, particularly cryptococcosis, along with the corresponding increase globally in the population of immunocompromised persons, creates a great demand for fast and inexpensive diagnostic methods.
The CrAg LFA test is likely to be adopted into resource-poor settings as it is cost-effective and simple. It will also be expanded to regions of the highest burden, especially in Sub-Saharan Africa and Asia-Pacific, where cryptococcosis remains an important cause of morbidity and mortality.
If sustained, the support from international health agencies, allied to that of governments, in the uptake of CrAg LFA would result in early diagnosis and timely treatment, leading to reduced deaths from fungal diseases. This makes it a perfect opportunity for improving public health globally-in vulnerable populations.
Increased inclusion of this test in international diagnosis guidelines for the diagnosis of fungal diseases attribute to the growth of the market. With growing awareness regarding repercussion of fungal diseases in immunocompromised patients, the demand for fast diagnostics tools such as CrAg LFA has been increasing.
Among industry stakeholders, diagnostic companies and public health agencies are propelling CrAg LFA because of its promise for improving early diagnosis of cryptococcal meningitis, which is usually a fatal product of HIV/AIDS. Increased interest in low-cost diagnostics for low-resource settings has formed strong ties between such stakeholders as government, NGOs, and mainstream international health organizations.
This coupled with new enhancements in manufacturing technologies has also led to a lot of scalability in CrAg LFA, allowing it to be made available in the high and low income regions alike. This marks a great milestone since it is testimony to the importance of the test in enhancing patient outcomes through timely intervention.
An emerging trend to the uptake of CrAg LFA testing will be the increasing interest in incorporating CrAg LFA into point-of-care (POC) facilities, especially in resource-poor settings. This evident drive towards the widening diagnosis access for suspected fungal diseases on the global health scale has already set grounds for the use of rapid tests such as CrAg LFA at the first point of patient access, i.e., community health centres, rural clinics and mobile health units.
This motivating factor for the trend to urgency for faster results and earlier intervention is that conditions like cryptococcal meningitis progress at exceptionally fast rates. Besides, mobile applications coupled with technology in digital data monitoring would help underpin this trend by real-time delivery and tracking of the patient.
These trends go in tandem with the current endeavors aimed at decentralizing health services, thus making diagnostics more affordable, available, and effective. The inclusion of CrAg LFA into standard healthcare, mainly in underprivileged quarters, will probably become one of the biggest achievements toward reducing global mortality from fungal infections.
The Cryptococcal Antigen Lateral Flow Assay (CrAg LFA) test is increasingly important for medical care because of rising incidences and cases of cryptococcosis, particularly in the immunocompromised individuals. This test has indeed provided a fast, precise and cost-effective means of diagnosing cryptococcal meningitis in the large population of immunocompromised patients with HIV/AIDS. There is a growing demand for CrAg LFA mostly in resource-poor countries because of the increasing awareness about the need for earlier diagnosis and treatment in such populations.
The other major driver of the growth that CrAg LFA will realize in its market development is in the long-term cost-effectiveness of such a product. Although the cost may be slightly high at initial procurement of the tests, the improved speed and ease of use along with provision of results at point of care make it handy for use by healthcare professionals. With continued improvements in technology and more global health efforts, the use of CrAg LFA will rise, making it an integral part of the health infrastructure in regions with high fungal infection burden.
Shifts in the Cryptococcal Antigen Lateral Flow Assay Test Market from 2020 to 2024 and Future Trends 2025 to 2035
| Category | 2020 to 2024 Trends |
|---|---|
| Regulatory Landscape | Regulatory bodies have increased focus on ensuring the safety and efficacy of diagnostic tools like CrAg LFA, especially for use in resource-limited settings. |
| Technological Advancements | Integration of mobile technology to assist in tracking test results and patient data, along with improvements in test sensitivity. |
| Consumer Demand | Growing demand driven by increasing awareness of fungal infections, especially in immunocompromised populations like those with HIV/AIDS. |
| Market Growth Drivers | Increased recognition by healthcare providers of the importance of rapid diagnostics for cryptococcal meningitis, especially in regions with high HIV prevalence. |
| Sustainability | Manufacturers focus on creating more affordable and sustainable diagnostic products, improving accessibility in low-resource areas. |
| Category | 2025 to 2035 Projections |
|---|---|
| Regulatory Landscape | Regulatory standards will become more stringent, requiring advanced testing validation, enhanced safety protocols, and greater oversight on production. |
| Technological Advancements | AI-driven diagnostics and real-time health monitoring tools integrated with CrAg LFA will enable personalized treatment plans and enhanced patient care. |
| Consumer Demand | Adoption will expand globally with a stronger focus on rapid, accurate diagnostics in decentralized care settings, addressing broader healthcare needs. |
| Market Growth Drivers | The rising burden of immunocompromised patients and expanding healthcare access will drive further demand for CrAg LFA tests in both urban and rural areas. |
| Sustainability | The industry will prioritize eco-friendly production methods, including reusable diagnostic components and sustainable materials. |
Increases in immunocompromised population due to HIV/AIDS, cancer therapies, or organ transplants offer the impetus for implementing CrAg LFA in the USA. Thus, the early detection of these infections, especially among at-risk populations, has gotten increasing attention in the USA.
Added to that, healthcare has focused on improving diagnostics that give rise to rapid diagnostics CrAg LFA as a tool that could not be missed in emergency care. Strong market prospects remain in the light of enhancing investments into healthcare technologies and public health initiatives aimed to fuel uptake of CrAg LFA in the years ahead
Market Growth Factors
| Country | CAGR (2025 to 2035) |
|---|---|
| United States | 3.9% |
Market Outlook
An advanced health care system and patient-oriented programs promoting the early diagnosis of infections are in support of the implementation of the CrAg LFA test in Germany. The German health system is precise, with special emphasis on optimizing clinical outcomes for immunocompromised patients, HIV/AIDS patients in particular. Further driving the need for CrAg LFA is an increasing awareness of fungal infections and their contribution to health care need for an aging population along with rapid advancement in diagnostic technologies. The future indeed looks bright as more health care providers promote the rapid point of care tests for fungal infections.
Market Growth Factors
Market Forecast
| Country | CAGR (2025 to 2035) |
|---|---|
| Germany | 4.3% |
Market Outlook
Growing HIV/AIDS and other immunocompromised states give a very urgent requirement of effective diagnostics such as CrAg LFA in India. Major challenges in the health care sector seem to be availability and access to specialized diagnostic tools, especially in rural settings. The CrAg LFA fits these requirements beautifully in terms of being economical and providing rapid results to India's health sector, where an early diagnosis for an opportunistic fungal infection could mean death or life. With the increasing emphasis on health care access and public health, the look ahead for CrAg LFA appears bright, further implored by the support of governance and international agencies for the control programs for fungal diseases.
Market Growth Factors
Market Forecast
| Country | CAGR (2025 to 2035) |
|---|---|
| India | 5.7% |
CrAg LFA is greatly propelled by China's enormous population and ever-rising numbers of immunocompromised, especially with elderly populations and higher cancer incidence. The demands for rapid, cost-efficient diagnostics arise from continuing healthcare reforms in China, aimed at strengthening diagnostic capabilities in urban and rural settings alike. Besides, with the strengthening of the health system to cope with infectious diseases, the CrAg LFA is gaining momentum as an important tool for early diagnosis and treatment of fungal infections. Strong growth is expected for CrAgLFA with the ever-increasing demand for diagnostics technology.
Market Growth Drivers
| Country | CAGR (2025 to 2035) |
|---|---|
| China | 6.1% |
The demand for a real diagnostic tool like CrAg LFA in Japan is driven by elderly and immuno-compromised patients such as aged and cancer patients. Japanese healthcare, which maintains the highest standards, focuses strongly on precision medicine and early disease detection, so CrAg LFA will be very much an important diagnostic method relevant to cryptococcal infections. Market prospects for CrAg LFA seem to be bright as far as innovations in healthcare technologies continue to be pursued further by Japan, and increasing further into urban and rural reachability as awareness increases among healthcare providers.
Market Growth Drivers
| Country | CAGR (2025 to 2035) |
|---|---|
| Japan | 5.1% |
The Critical Role of Lateral Flow Readers in Ensuring Accurate and Reliable Result aid it to hold Dominant Position in the Market
Lateral Flow Readers perform the essential functions of reading results from lateral flow tests in a standardized and automated way so that human errors are minimized, and reproducibility in clinical settings is maximized. Therefore, in a conjoined action toward quick- and accurate-diagnostic solutions, these lateral flow readers are by now being integrated into various healthcare settings.
With simultaneous processing of multiple tests along with ease of usability, scalability, and low cost, these readers have become an indispensable asset in hospitals, clinics, and mobile testing sites, giving them leadership in the market.
Growing demand for kits and Reagent across Different Healthcare Settings aid them to Grow at Higher Rate
The Kits and Reagents segment expanding with the highest CAGR due to increasing demand for CrAg LFA tests in various healthcare settings. These kits contain all the components, such as antigen reagents, buffers, and detection materials, required to conduct the test; therefore, they are extremely vital in making CrAg LFA available across large numbers of settings.
Increasing worldwide adoption of point-of-care diagnostics and a trend toward cost-effective options have raised the demand for kits that are easy to deploy. Ongoing advancements in reagent compositions with improved sensitivity and accuracy would continue to spur the segment's growth and propel greater usage in limited-resource settings and mobile health units.
The Growing Requirement for Rapid and Accurate Diagnostic Tools in Hospitals aid it to Hold Highest Market Share
Hospitals are at the center of care for high-risk patients, especially those that are immunocompromised, such as HIV/AIDS patients or patients who have undergone organ transplantation. Effective, trustworthy diagnostic devices like CrAg LFA are required by hospitals for the prompt detection of cryptococcal meningitis, which often leads to severe problems if unattended or even death.
Furthermore, hospitals have more patients and advance infrastructure, thus presenting itself as an ideal setting for the major use of CrAg LFA tests. Moreover, the increased number of admissions for patients who are immunocompromised into hospitals also favors the need for these diagnostics, thus maintaining the dominance of the hospital segment.
The Rising Demand for point-of-care diagnostics from Ambulatory Clinics aid it to gain Traction in the Market
Ambulatory clinics, which provide outpatient care, are growing at the fastest CAGR for several reasons. Increased demand for point-of-care tests like CrAg LFA has been experienced in ambulatory clinics over the years. In fact, HIV/AIDS patients accessing these clinics are at risk of cryptococcal infections, hence the need to have quick and less expensive diagnostics for early intervention.
The technique that CrAg LFA employs to provide rapid and accurate results without the necessity for expansive laboratory setup is perfect for ambulatory clinics. Moreover, the increasing amount of decentralization and availability of diagnostic services worldwide beyond hospitals is quite relevant in arguing that ambulatory clinics would adopt these tests, hence a rapid expansion of the segment.
The new competition in the market about this innovative test-the cryptococcal antigen lateral flow assay (CrAg LFA) keeps launching new entrants. The current thread is that the diagnostic companies develop and put great investment in the CrAg LFA to meet the increasing point-of-care demand for faster tests that are accurate and easy to conduct, especially in resource-constrained environments.
Intensifying the competition also includes manufacturers who emphasize the signature characteristics of their products, particularly increased sensitivity, cost-effectiveness, and integration into digital health systems. With increased sensitivity to fungal infections in these high-risk groups, especially for people living with HIV/AIDS, access and price have also featured a more significant emphasis on increased availability..
Market Share Analysis by Company
| Company Name | Estimated Market Share (%) |
|---|---|
| Kestrel Biosciences LIC | 32.5% |
| Alere Inc | 20.6% |
| BioMerieux | 17.0% |
| Danaher Corporation | 6.8% |
| Other Companies (combined) | 23.0% |
| Company Name | Kestrel Biosciences LIC |
|---|---|
| Year | 2024 |
| Key Company Developments and Activities | Market leader providing high-containment HPOSD manufacturing, including highly potent APIs and finished dosage forms. |
| Company Name | Alere Inc |
|---|---|
| Year | 2024 |
| Key Company Developments and Activities | Offers specialized development and manufacturing services for high-potency tablets and capsules, focusing on scalable production. |
| Company Name | BioMerieux |
|---|---|
| Year | 2024 |
| Key Company Developments and Activities | Provides comprehensive HPOSD solutions, including formulation development, analytical services, and commercial production. |
| Company Name | Danaher Corporation |
|---|---|
| Year | 2024 |
| Key Company Developments and Activities | Develops complex HPOSD formulations with a focus on containment technologies and regulatory compliance. |
Key Company Insights
Galderma SA
Galderma, the front-runner in global cosmetic surgery products, specializes in dermatological and aesthetic solutions. Galderma is a pioneering name that came out with injectable fillers and botulinum toxins and advanced skincare for medical and cosmetic applications.
Johnson & Johnson Services
As one of the world's biggest healthcare conglomerates, it is represented in aesthetics and reconstructive surgery by two subsidiaries: Mentor Worldwide, which is breastimplant-focused, and Ethicon, which is known for wound closure and skin-tightening technologies. Research and development cost consummate most of the investments which push the technologies in the domain of cosmetic surgery.
Merz Pharma GmbH & Co. KGaA
It ranges from medical aesthetics to neurotoxins. Merz Pharma has a very solid position in the arena of cosmetic surgery, endowed with an extensive botulinum toxin, dermal fillers, and antiaging product portfolios, all streamlined within penetrating levels within the USA and European markets.
Cynosure LLC
Being one of the largest healthcare conglomerates, this company forays into aesthetic and reconstructive surgery through its subsidiaries Mentor Worldwide, which focuses on breast implants and Ethicon, known for its technologies on wound closure and skin-tightening. Heavy investments in R&D take the lead in the advancement of technologies in cosmetic surgery.
Beyond the leading companies, several other contract manufacturers contribute significantly to the market, enhancing service diversity and technological advancements. These include:
Key Company Insights
Kestrel Biosciences LIC: Kestrel Biosciences LIC is devoted to the manufacture of high-performance Cryptococcal Antigen Lateral Flow Assay tests, offering express and accurate results. The new technologies developed increase test sensitivity and specificity, making them appropriately suited for clinical and field use, particularly for immunocompromised patients.
Alere Inc: Alere Inc. specializes in diagnostics, is committed to Cryptococcal Antigen Lateral Flow Assay testing being accessible and affordable. Their efforts in speeding turnaround time and user simplicity empower healthcare providers to efficiently treat cryptococcal infections, very much making a difference in resource-challenging areas, where rapid treatment can save lives.
BioMerieux: The principal focus of BioMerieux in survey CrAg-LFA test states that their main characteristics are accuracy, rapidity, and practicality. Technological advances aim to produce accurate and prompt diagnostics of fungal diseases in order to improve the health of patients, especially those who are immunocompromised.
Danaher Corporation: Danaher Corporation is a world leader in diagnostic solutions, developing Cryptococcal Antigen Lateral Flow Assay tests through innovations that aim to enhance sensitivity and shorten detection time. Thus, with its intention of enhancing global affordability, its very affordable tests will be essential in clinical and POCT settings around the globe.
Beyond the leading companies, several other manufacturers contribute significantly to the market, enhancing product diversity and technological advancements. They include:
These companies focus on expanding the reach of cryptococcal antigen lateral flow assay test, offering competitive pricing and cutting-edge innovations to meet diverse needs.
The overall market size for cryptococcal antigen lateral flow assay test market was USD 796.8 million in 2025.
The cryptococcal antigen lateral flow assay test market is expected to reach USD 1,149.1 million in 2035.
Increase in number of people suffering from immunocompromised diseases anticipates the growth of the market.
The top key players that drives the development of cryptococcal antigen lateral flow assay test market are Kestrel Biosciences LIC, Alere Inc, BioMerieux, Danaher Corporation and Qiagen.
Lateral flow readers segment by instruments is expected to dominate the market during the forecast period.
Lateral Flow Readers and Kits and Reagents
Home Testing, Point of Care Testing and Laboratory Testing
Diagnostic Laboratories, Ambulatory Clinics, Home Healthcare and Hospitals
North America, Latin America, Western Europe, Eastern Europe, East Asia, South Asia & Pacific, Middle East & Africa
CGRP Inhibitors Market Trends - Growth, Demand & Forecast 2025 to 2035
Indolent Systemic Mastocytosis treatment Market Insights: Size, Trends & Forecast 2025 to 2035
Intraoperative Fluorescence Imaging Market Report - Demand, Trends & Industry Forecast 2025 to 2035
Cardiovascular Diagnostics Market Report- Trends & Innovations 2025 to 2035
Venous Ulcer Treatment Market Overview - Growth, Trends & Forecast 2025 to 2035
Leukocyte Adhesion Deficiency Management Market - Innovations & Treatment Trends 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.