The following new report is now available, a Global CRISPR and Cas Gene Market: Analysis by Product, Application, Region, Size and Trends with Impact of COVID-19 2025 to 2035: Market Insights This can be attributed to need for development in technologies of gene-editing, wide applications, expansion of commercial and industrial scale gene-editing, and rising demand for precision medicine.
CRISPR clusters of regularly interspaced short palindromic repeats and their associated Cas genes have rewritten the rules of genetic engineering with precise editing of DNA sequences. Key factors driving global market growth include increasing CRISPR adoption for applications such as gene therapy, disease modeling, and agricultural biotechnology, along with advancements in genome sequencing.
Furthermore, the emergence of synthetic biology, acceleration in investment in biotechnology research, and increasing regulatory endorsement towards gene-editing therapies are additionally playing important role in the burgeoning industry.
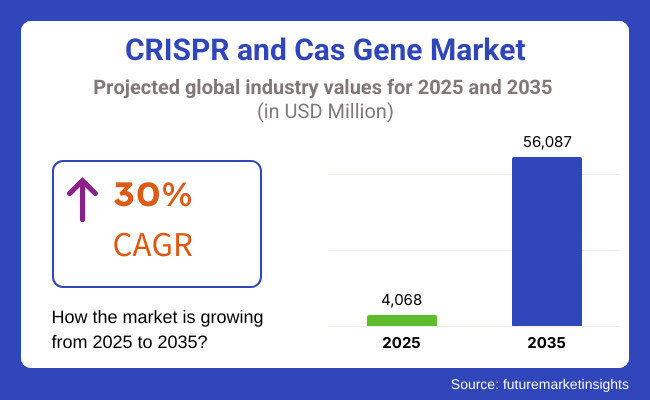
The CRISPR and Cas gene market was worth around USD 4,068 Million in 2025. The Global Cloud Kitchen Market size was estimated at USD 16,917 Million in 2022 and is projected to reach USD 56,087 Million by 2035, exhibiting a CAGR of 30%. This market is driven by the rising demand for genetic modifications in healthcare and agriculture, increasing prevalence of genetic disorders, and growing investments in personalized medicine.
The market growth is further driven by the incorporation of AI-powered bioinformatics to enhance CRISPR delivery systems as well as cost-effective gene-editing plants. Moreover, the increased penetration of the market and adoption of the industry is mostly attributable to the improved safety, regulatory-approved CRISPR-based therapy and high-precision gene editing techniques.
Explore FMI!
Book a free demo
Check this also: North America is a leading region for CRISPR and Cas gene technologies market owing to its strong biotechnology research, high use rates of gene-editing tools, and large genomics investments. Leading the world in developing and commercializing next-generation CRISPR-based therapies and applications in cancer treatment, unusual genetic disorders and regenerative medicine, are the United States and Canada.
These factors along with rising interest in precision medicine, approval of clinical trials based on gene-editing technologies and rise in funding for genetic research is further driving the market growth. Moreover, product innovation and adoption are also being promoted by the rapid growth of AI-driven CRISPR design tools and bioinformatics platforms.
The growth of CRISPR technology, government and industry-led research projects for genome editing, and rising adoption trends for genome editing in healthcare and agriculture are all contributing factors to the market growth for Europe. Germany, France and the UK, for example, are all pursuing high-precision, ethically regulated applications of gene-editing technology for medical and agricultural purposes.
The increasing focus on rare disease therapies, expanding applications in genetically modified crops, and research in CRISPR-based diagnostics will also drive market adoption. Moreover, new applications in pharmaceutical drug development, regenerative medicine, and cell therapy are opening up new avenues for biotech firms and research organizations.
Such factors such as increased government support of genetic research, increasing demand for innovative therapeutics and growing investments in biotechnology startup make the Asia-Pacific region the leading contributor in terms of growth rate for the CRISPR & Cas Gene Market. Again, countries you mention: China, India, and Japan these countries are investing heavily in gene-editing research, CRISPR-based agricultural solutions, and personalized medicine.
The increasing adoption of advanced genetic therapies, demand for ecosystem expansion of genome sequencing projects, changing research and manufacturing regulatory environment, and government initiatives in supporting biotech innovations is driving the regional market expansion.
Moreover, the growing awareness about gene therapy applications and CRISPR-based drug development has also supported the market penetration. Additionally, the growing number of domestic biotech companies and collaboration with international pharmaceutical companies could be contributing to the market growth.
With the growing prevalence of genetic research and the increasing attention on biotechnological advancements along with the growing investments in precision medicine, the Latin American market is sustaining healthy growth. Brazil and Mexico are the leading contributors putting their efforts on making CRISPR-based gene-editing solutions available for medical and agricultural usage.
The usage of local biotech innovations at their disposal, affordable research programs, and gene-editing awareness promotional programs is aiding in the growth of the market. Moreover, growing access to clinical trials for gene therapies, increased funding of genetic engineering, and demands from consumer-driven markets for genome-related healthcare services, are making access easier throughout the region.
The Middle East & Africa contributes a gradually increasing share to the CRISPR and Cas genes market, supported by rising investments in biotechnology, genomic studies, and precision medicine endeavors. This initiative is founded on the introduction of the UAE and South Africa to optimize product availability and technologies.
Market growth is further propelled by a surge in research on genetic diseases, high demand for precise gene-editing tools, and collaboration between international and local biotech enterprises. Longer-term industry growth is being bolstered by government policies in favor of genetic research, innovations in CRISPR-based agriculture and consumer-driven demand for advanced healthcare solutions.
The growth of the market is being supported by the rising initiatives of global biotech collaborations as well as the growing presence of genome-editing start-ups in the regional market.
The continuous research and development of CRISPR technology, synthetic biology, and personalized medicine suggest that the CRISPR and Cas gene market is likely to expand exponentially over the next ten years. These innovations in gene-editing delivery systems, AI-enabled genetic modification tools, and precision genome engineering promise enhanced functionality, commercial appeal, and enduring use of engineered products.
And the future of the industry is being shaped by factors like growing consumer interest in genetic research, the digitalization of biotechnology and changing ethical regulations. AI-driven CRISPR design, next-generation sequencing innovations, and regulatory-compliant gene therapies are already optimizing research efficiency and ensuring high-quality gene-editing applications worldwide.
Challenge
Ethical and Regulatory Concerns
Moreover, CRISPR one of the most relevant technologies in the gene market has received attention on in vitro/-ex vivo or in vivo applications (to some extent homologous recombination to introduce/delete genes). With a strict set of FDA, EMA, and WHO imposed guidelines regarding gene-editing applications, research and commercialization is suffocated.
The fear of unintended genetic consequences, bioethics, and potential misuse make market expansion more complicated. Companies need to proactively engage in transparent scientific communication, adhere to bioethical norms across the globe, and collaborate with regulatory agencies to ensure responsible CRISPR bio-agents development and application.
Off-Target Effects and Delivery Challenges
Although CRISPR is highly precise, the risk of making unintended genetic changes elsewhere in the genome is a lingering concern for therapeutic use. Inadvertent genetic modifications can result in unpredictable mutations, which can have negative consequences. Moreover, the technical hurdles associated with the efficient and safe delivery mechanisms for CRISPR components (E.g. Viral vector; Lipid nanoparticle, etc.) also persists.
The lack of delivery systems that are highly specific severely limits CRISPR’s effectiveness in clinical applications. They will need to develop advanced bioinformatics tools, new AI-driven off-target prediction models, and innovative delivery technologies that will underpin safer and more precise CRISPR.
Opportunity
Expansion of Gene Therapy and Personalized Medicine
This is a major opportunity for the CRISPR and Cas gene market as there is an increasing demand for personalized medicine and gene therapy. CRISPR-based gene editing allows for precise interventions, as seen with genetic disorders like sickle cell disease, cystic fibrosis and muscular dystrophy.
In addition, new autologous and allogeneic cell therapies are transforming cancer therapy. The next generation of healthcare innovation will be led by companies focused on CRISPR-based precision medicine, cell therapy innovations, and regenerative medicine applications.
Agricultural and Industrial Applications of CRISPR
Outside of healthcare, CRISPR technology is being used increasingly in agriculture and industrial biotechnology. Genetic modification that yields crops with more resistance to pests, diseases, and environmental stress is consequently enhancing global food security. Synthetics: CRISPR is found in the bioengineering sector, which helps optimize biofuel production, bioplastics, and enzyme production.
Genetics Technologies - Sustainable CRISPR Applications for Agriculture, Synthetic Biology & Industrial Biotechnology Companies investing in sustainable CRISPR applications for agriculture, synthetic biology, and industrial biotechnology will be able to capitalize on new growth opportunities in the growing gene-editing ecosystem.
Market Dynamics: CRISPR & Cas Gene Market (2025 to 2035) Based on 2020 to 2024 Market Trend. CRISPR and Cas gene market had observed growth from 2020 to 2024 and is expected to provide various opportunities for other players, researchers, and patients involved in the healthcare and biotechnology sectors. Companies targeting improvements in CRISPR editing efficiency, off-target effects, and gaining regulatory clearance for clinical trials.
Nonetheless, issues like ethical implications, patent barriers, and low commercial availability of CRISPR-derived therapies, stifled generalization. Among other measures, businesses reacted by reinforcing bioethics policies, advancing regulatory compliance strategies, and expanding collaborations with academic and pharmaceutical entities.
Next steps will be covered in terms of in vivo gene editing, AI-Enabled CRISPR design and next-gen Delivery. Base editing, prime editing, and epigenome-targeting CRISPR tools will change gene therapy as we know it. And integration of CRISPR with AI-based drug discovery approaches, biofoundries, and synthetic biology platforms will even further accelerate innovation.
The next era of CRISPR-led biological and medical innovation will be led by companies with safety, precision and interdisciplinary collaboration at their core.
| Market Shift | 2020 to 2024 Trends |
|---|---|
| Regulatory Landscape | Strict regulations on human gene editing and ethical concerns |
| Technological Advancements | Growth in CRISPR-based diagnostics and ex vivo gene editing |
| Industry Adoption | Increased use in rare disease treatments and research applications |
| Supply Chain and Sourcing | Dependence on academic research institutions and biotech startups |
| Market Competition | Dominance of biotech firms specializing in gene editing |
| Market Growth Drivers | Demand for precision medicine and genetic disease treatments |
| Sustainability and Energy Efficiency | Initial focus on improving gene-editing efficiency and scalability |
| Integration of Smart Monitoring | Limited real-time tracking of gene-editing outcomes |
| Advancements in Gene Editing Innovation | Development of base editing and prime editing |
| Market Shift | 2025 to 2035 Projections |
|---|---|
| Regulatory Landscape | AI-driven regulatory compliance, global harmonization of gene-editing policies, and enhanced bioethical oversight |
| Technological Advancements | Expansion of in vivo CRISPR therapies, AI-powered genome editing, and next-generation precision gene repair |
| Industry Adoption | Widespread adoption in regenerative medicine, oncology, and neurodegenerative disorder therapies |
| Supply Chain and Sourcing | Integration of CRISPR into large-scale pharmaceutical manufacturing, synthetic biology, and decentralized biofoundries |
| Market Competition | Rise of AI-driven gene-editing companies, CRISPR-based synthetic biology startups, and pharmaceutical collaborations |
| Market Growth Drivers | Expansion of CRISPR in agriculture, industrial biotechnology, and biomanufacturing for sustainable solutions |
| Sustainability and Energy Efficiency | Large-scale implementation of CRISPR for climate-resilient crops, biofuel optimization, and synthetic ecosystem engineering |
| Integration of Smart Monitoring | AI-powered CRISPR diagnostics, real-time genetic error detection, and blockchain-enabled genomic data security |
| Advancements in Gene Editing Innovation | Introduction of epigenetic CRISPR tools, self-regulating gene circuits, and next-gen RNA-targeting therapies |
Due to heavy government support for genomic research, growing applications in gene therapy, and the presence of several major biotech companies, the US controls the CRISPR and Cas gene market. Growth in the market continues to be propelled by rapid advances in gene editing technologies and the increasing interest in personalized medicine.
An increase in investments in CRISPR-based therapeutics, coupled with advancements in high precision gene editing tools, is expected to bolster the market growth. Moreover, AI-driven genome sequencing is being merged with synthetic biology applications while CRISPR-based diagnostics are advancing yet further treatment possibilities.
Next-generation CRISPR, such as base and prime editing, is also a focus of companies seeking to improve CRISPR technology regarding accuracy and efficiency. Moreover, the growing adoption of CRISPR for disease modeling, drug discovery, and agricultural biotechnology is also propelling the USA market demand.
| Country | CAGR (2025 to 2035) |
|---|---|
| USA | 31% |
High investments in gene-editing research, increasing collaborations between academia and biotech firms, and rising regulatory approvals for genome-based treatments are boosting the CRISPR and Cas gene technology market in United Kingdom. Increasing focus on therapies for rare diseases is also propelling market growth.
The growth of the market during the forecast period is buoyed by various government supports in parallel with the emergence of CRISPR migraine diagnosis and regenerative medicine. Additionally, being built up are advances in non-viral gene delivery techniques; synthetic guide RNAs; and techniques to reduce off-target editing.
Enterprises are also putting money into ex vivo gene editing platforms for cell and gene therapies. Additionally, the growing emphasis on developing ethical and regulatory frameworks for gene editing along with advances in CRISPR-based agriculture is propelling the market expansion in the UK. Moreover, the increasing use of precision medicine and cancer immunotherapy applications are driving the demand for CRISPR solutions.
| Country | CAGR (2025 to 2035) |
|---|---|
| UK | 29.5% |
Germany, France, and Italy are the leading nations in the European CRISPR and Cas genes market, owing to robust research infrastructure, growing biotech investments, and conducive regulatory environments that promote the advancement of gene editing.
Rapid market growth is also promoted by the European Union's efforts to promote genetic research and investments in CRISPR-based therapies for genetic disorders. CRISPR has led to progress in functional genomics, stem cell research, and precision agriculture, further enhancing product innovation.
The market is further driven by increasing demand for next-generation CRISPR platforms for drug discovery and regenerative medicine. The broader adoption across the EU is being bolstered by the extension of ethical guidelines on gene editing and the establishment of regulatory policies on genome editing. Additionally, funding programs for biotech startups and genome engineering efforts are accelerating the commercialization of CRISPR-based solutions.
| Country | CAGR (2025 to 2035) |
|---|---|
| European Union (EU) | 29.8% |
Japan CRISPR and Cas Gene Market is expanding, owing to the country strong focus on genetic engineering, increasing applications in regenerative medicine, and advancements in stem cell research. Increasing demand for targeted gene therapeutics development and disease-resistant crops.
Combine this with the country’s strong focus on precision medicine and the convergence of AI-enhanced genome editing and CRISPR-derived cellular therapies, and innovation is booming. Additionally, stringent government regulations related to gene-editing applications in humans and agriculture have been creating the impetus for the companies to develop high-safety and ethically compliant CRISPR solutions.
The growing prevalence and demand for CRISPR-based treatments across various clinical areas such as oncology, inherited disorders, and neuroscience is also speeding up market growth within Japan's biotech sector. Japan’s investment in synthetic biology and genome-wide screening technologies is also one of the earliest applications of gene editing for the future.
| Country | CAGR (2025 to 2035) |
|---|---|
| Japan | 30.2% |
The market is expected to grow significantly in South Korea, fueled by increasing biotechnological research initiatives, adoption of CRISPR in the precision medicine sector, and government-financed genome-editing projects.
Favorable government policies supporting genetic research, combined with investments in CRISPR-based drug development and gene therapy development, make way for the market growth. And at the same time, that push to improve gene-editing precision - including a focus on computational biology powered AI, synthetic RNA engineering, and CRISPR-Cas variant optimization - is bolstering competitiveness.
Increasing commercialization of CRISPR in agricultural biotechnology, functional genomics, and industrial microbiology will also drive demand for this market. Businesses have been making investments in new CRISPR technologies to treat hereditary diseases, engineer tissues, and manufacture biopharmaceuticals. Increased collaborations with global genomic research institutes and emergence of biotech startups have propelled demand for CRISPR-based innovations in South Korea.
| Country | CAGR (2025 to 2035) |
|---|---|
| South Korea | 30% |
Kits & enzymes are the significant components of the CRISPR and Cas gene as they help in genome editing, DNA cleavage along with gene expression modifications. Modeling diseases, building functional genomics, and developing therapies require high-precision CRISPR kits, which are mostly purchased by academia and biotechnology companies.
Recent developments in gene editing efficiency are largely driven by the increasing need for customizable, high-fidelity CRISPR-Cas9 enzyme systems and pre-assembled ribonucleoproteins (RNPs). Further development of CRISPR-associated (Cas) enzyme variants, such as Cas12 and Cas13, have diversified this platform beyond traditional gene editing applications into diagnostic and therapeutic arenas.
CRISPR libraries are a key component for high-throughput genomic studies, allowing for genome-scale screens in functional studies, drug development, and target identification. These libraries represent estarge collections of guide RNA (gRNA) that enable systematic experiments for gene knockout, knock-in or activation.
The increasing demand for personalized medicine, cancer studies, and precision genome alterations has driven the adoption of whole-genome and custom gRNA libraries. Moreover, the combination of CRISPR screening tools driven by AI and cloud-based data analysis platforms can greatly enhance the reproducibility of experiments and accelerate research progress.
Engineering of cell lines continues to be a leading service segment in the CRISPR and Cas gene space, as these tools will support biopharma research as well as applications utilizing regenerative medicine and synthetic biology. CRISPR technology is being used by scientists to generate genetically modified cell lines for disease modeling, antibody production, and protein expression studies.
Moreover, the growing application of CRISPR-edited stem cells and patient-derived organoids in translational research has further propelled the demand for precise gene knockout, knock-in, and multiplex genome editing services. Moreover, implementation in gene therapy and supportive regulations for regenerative medicine approvals have fueled investments in custom tailored cell line development.
The need for gRNA (guide RNA) design services, as they are responsible for CRISPR optimization to avoid off-target effects and higher specificity. Predicting gRNA sequence, characterizing mismatch tolerance and modeling secondary structure have greatly improved through the development of computational bioinformatics tools, which have also enabled the achievement of highly efficient editing events.
The growing need for customized gene editing solutions in oncology, rare diseases, and agricultural biotechnology has accelerated automated and artificial intelligence-powered gRNA design platforms. Deep learning-based gRNA scoring and synthetic biology inputs truly revolutionized the precision and scalability of gene editing initiatives.
The clinical application segment is accelerating due to its growing adoption in genetic disorders, oncology, and infectious disease therapy. Breakthroughs in the treatment of sickle cell disease, beta-thalassemia, and hereditary blindness have come about with advances in ex vivo and in vivo gene editing approaches.
Growing Utilization of CRISPR-driven Drug Discovery Pipelines, Gene Correction Therapies, and Cell-based Immunotherapies by Pharmaceutical Companies Driving Market Growth The entrance of CRISPR-mediated diagnostics, including SHERLOCK and DETECTR, has extended the technology's clinical reach into point-of-care genetics screening and rapid pathogen detection .
CRISPR Technologies could be used in Operational & administrative applications include agriculture biotechnology, biosecurity monitoring, and precision livestock breeding. In the agrigenomics sector, crops with resistance to diseases using the CRISPR approach, higher yield potential, and without compromising on the nutritional quality have already been gaining some degree of traction.
The need for the means of genome editing in livestock genetics, pest control, and sustainable food production has led to investments in CRISPR-based agricultural warfare programs. Besides, it enables optimization in scalability of research and the efficiency of biopharmaceutical production through automated laboratory workflows, high-throughput screening, and robotic genome editing platforms.
The CRISPR and Cas Gene market is the result of good research approach, development of machinery and more innovative products to cater the growth in demand. The CRISPR market also is becoming more crowded as companies focus on efficient therapies, expand genome engineering capabilities, and improve delivery for precision medicine. Important trends highlighted in this research report include an upsurge in CRISPR-based drug discovery, gene therapy applications, and advanced off-target editing solutions.
Market Share Analysis by Company
| Company Name | Estimated Market Share (%) |
|---|---|
| Editas Medicine, Inc. | 18-22% |
| CRISPR Therapeutics AG | 14-18% |
| Intellia Therapeutics, Inc. | 11-15% |
| Beam Therapeutics | 8-12% |
| Caribou Biosciences, Inc. | 6-10% |
| Other Companies (combined) | 30-40% |
| Company Name | Key Offerings/Activities |
|---|---|
| Editas Medicine, Inc. | Leading developer of CRISPR-based gene therapies targeting genetic disorders. |
| CRISPR Therapeutics AG | Specializes in CRISPR-based treatments for blood disorders, oncology, and regenerative medicine. |
| Intellia Therapeutics, Inc. | Focuses on in vivo CRISPR gene editing therapies for rare diseases. |
| Beam Therapeutics | Pioneers in base editing technology for precision gene modification. |
| Caribou Biosciences, Inc. | Develops CRISPR-based solutions for agricultural and therapeutic applications. |
Key Company Insights
Editas Medicine, Inc. (18-22%)
Editas Medicine is emerging as a heavyweight key player of CRISPR, working on therapy development of the gene editing for inherited genetic disorders.
CRISPR Therapeutics AG (14-18%)
CRISPR Therapeutics is focused on developing transformative CRISPR-based therapies for serious diseases.
Intellia Therapeutics, Inc. (11-15%)
Next-gen in vivo gene editing therapies to treat heritable, rare genetic conditions and chronic diseases
Beam Therapeutics (8-12%)
Top company in the industry: Beam Therapeutics - This company has developed base editing technology which allows us to precisely modify our genetics with low off-target effects.
Caribou Biosciences, Inc. (6-10%)
Caribou Biosciences develops CRISPR gene editing technologies with proprietary Cas enzymes, for therapeutic or agricultural use.
Other Key Players (30-40% Combined)
Global biotech companies and research institutes working on CRISPR and Cas genes focused on precision medicine, synthetic biology, or agricultural improvements. Key players include:
The overall market size for CRISPR and Cas gene market was USD 4,068 Million in 2025.
The CRISPR and Cas gene market expected to reach USD 56,087 Million in 2035.
Some of the key factors driving growth of the CRISPR and Cas gene market includes rising used in gene therapy and drug development, growing investments in biotechnology research, increasing prevalence of genetic disorders, advancements in precision medicine, and growing application of genome editing for agriculture and disease modeling.
The top 5 countries which drives the development of CRISPR and Cas gene market are USA, UK, Europe Union, Japan and South Korea.
Kits & enzymes and libraries growth to command significant share over the assessment period.
The Prurigo Nodularis (PN) Treatment Market is segmented by product, and end user from 2025 to 2035
Warm Autoimmune Hemolytic Anemia (WAIHA) Treatment Market Analysis by Drug Class, Distribution Channel, and Region through 2035
Atrophic Vaginitis Treatment Market Analysis And Forecast by Diagnosis, Treatment, Therapy Type, Distribution Channel, and Region through 2035
Adrenal Crisis Management Market Analysis and Forecast, By Diagnosis Method, Treatment Method, Distribution Channel, and Region, through 2035
Birch Allergy Treatment Market Analysis by Drug Class, Route of Administration, Distribution Channel and Region: Forecast from 2025 to 2035
Cancer Vaccines Market Analysis by Technology, Treatment Method, Application and Region from 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.