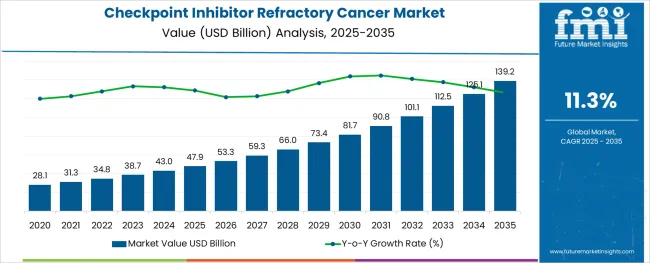
The Checkpoint Inhibitor Refractory Cancer Market is estimated to be valued at USD 47.9 billion in 2025 and is projected to reach USD 139.2 billion by 2035, registering a compound annual growth rate (CAGR) of 11.3% over the forecast period.
The checkpoint inhibitor refractory cancer market is witnessing significant momentum as resistance to standard immunotherapies emerges as a critical challenge in oncology. Advances in understanding tumor immune evasion mechanisms have created avenues for specialized therapies that address refractory cases, offering renewed hope for patients who do not respond to first-line checkpoint inhibitors.
Continued investment in clinical trials and biomarker-driven drug development is shaping the competitive landscape, with pharmaceutical companies focusing on combination regimens and novel targets beyond traditional pathways. Regulatory agencies are prioritizing accelerated approvals in this space, reflecting the unmet clinical need and strong demand from oncologists for more effective options.
In the coming years, the market is expected to benefit from expanding research into tumor microenvironment modulation, improved patient stratification techniques, and strategic alliances between biotechnology firms and academic research centers that are paving the path for more durable and personalized treatment solutions.
The market is segmented by Type and Application and region. By Type, the market is divided into PD-1 Inhibitor, CTLA-4 Inhibitor, PD-L1 Inhibitor, and Others. In terms of Application, the market is classified into Lung Cancer, Bladder Cancer, Melanoma, Hodgkin Lymphoma, and Others. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
Insights into the PD 1 Inhibitor Type Segment
When segmented by type, the PD 1 inhibitor segment is projected to command 42⋅5 share of the market in 2025, reinforcing its leadership position. This dominance has been supported by the well-established mechanism of action of PD 1 inhibitors, which has set the foundation for further development and refinement of therapies targeting refractory cases.
Widespread clinical familiarity and robust safety profiles have contributed to continued reliance on PD 1 based strategies, even in the face of resistance, often as a component of combination regimens. Advances in biomarker identification and dosage optimization have allowed for more precise application of PD 1 inhibitors in refractory populations, extending their utility beyond initial assumptions.
Pharmaceutical pipelines remain heavily concentrated on enhancing the efficacy of PD 1 inhibitors through adjunctive therapies, which has sustained investment and innovation in this segment, securing its leading share of the market.
Segmented by application, lung cancer is expected to hold 38⋅0 share of the checkpoint inhibitor refractory cancer market in 2025, establishing itself as the most significant application segment. This prominence has been reinforced by the high incidence and mortality associated with lung cancer, making it a critical focus area for therapeutic development.
The complex tumor biology and historically poor prognosis in refractory lung cancer cases have driven sustained clinical and commercial attention toward this indication. Enhanced screening programs and improved molecular characterization of lung tumors have enabled more targeted approaches, including refractory-specific interventions.
Additionally, strong engagement from both public and private sectors in funding lung cancer research has facilitated broader access to innovative treatments, fostering adoption of checkpoint inhibitor refractory therapies. The urgency to address unmet needs in this population has propelled lung cancer to the forefront of application areas within the market, consolidating its leadership position.
The global demand for Checkpoint Inhibitor Refractory Cancer is projected to increase at a CAGR of 11.26% during the forecast period between 2025 and 2035, reaching a total of USD 139.2 Billion in 2035, according to a report from Future Market Insights (FMI). From 2020 to 2025, sales witnessed significant growth, registering a CAGR of 5.5%.
According to Future Market Insights, a market research and competitive intelligence provider, the Checkpoint Inhibitor Refractory Cancer market was valued at USD 47.9 Billion in 2025.
The key factors leading to the market growth include the geriatric population across the globe, as well as a rapid increase in the global prevalence of several cancers, mainly in emerging and developed economies, which is expected to boost market growth in the forthcoming years.
For instance, as the World Health Organization reports, cancer has become a primary cause of death globally. As per the updated release of Globocan report released by the International Agency for Research on Cancer (IARC), in December 2024, the global burden of cancer has risen to 43 million cases and deaths by cancer have increased to 10 million in 2024. Thus, an upsurge in the volume of the patient pool leads to a greater demand for the market, which is accelerating the growth of the global market during the analysis period.
Leading industry players are focusing on the development and launch of novel PD-1/PD-L1 inhibitors to treat various types of cancers. This escalated the availability of PD-1/PD-L1 immunotherapeutic in the market is expected to boost the checkpoint inhibitor refractory cancer market.
Rise in Incidence of Cancer Globally to Fuel the Market Growth
The rise in the prevalence of cancer around the world is a major factor that will escalate the growth of the global checkpoint inhibitor refractory cancer market in the coming years. For instance, these checkpoint inhibitors are significantly used as front-line treatments for several types of cancer. As per the World Health Organization (WHO), cancer was one of the leading causes of death worldwide, accounting for around ten million deaths in 2024, or nearly one in six deaths. The most common cancers include breast, lung, colon and rectum, and prostate cancers. Thus, there is an increase in demand for checkpoint inhibitors globally.
There are various novel therapies, which have been approved in recent years. In addition, there is a gap between the demand and supply of these therapies, which has led to a huge unmet need. This, in turn, has given vendors an opportunity to conduct studies on drugs such as checkpoint inhibitors. In addition, the increasing approvals of these inhibitors are expected to increase their demand, which is anticipated to fuel the growth of the market.
Increase in Geriatric Population to Accelerate the Market Growth
The rise in cases of the geriatric population globally is projected to fuel the growth of the checkpoint inhibitor refractory cancer market over the analysis period. For instance, as per the Union for International Cancer Control (UICC), the elderly (people aged 65 years and more) are 11 times more likely to develop cancer, when compared to younger people. According to the United Nations, there are over 28.1 million people aged 65 or older, a number that is expected to reach 1.5 billion by 2050. According to the latest estimates and projections from UN DESA’s Population Division, one in six people in the world will be over the age of 65 by 2050, up from 1 in 11 in 2020.
The rise in research and development activities is expected to offer significant lucrative avenues for players in the checkpoint inhibitor refractory cancer market. For instance, market players have been increasingly investing in Research and Development activities, in order to meet the increasing demand. For instance, researchers at Johns Hopkins Kimmel Cancer Center are paving the way in developing novel immunotherapies called anti-PD-1 and anti-PD-L1 for people with advanced melanomas. The aim of the therapy is not to kill cancer cells directly, but to block a pathway, that protects tumor cells from components of the immune system that are capable and ready to fight cancer.
An increase in awareness among people about immune checkpoint inhibitors is projected to provide significant growth prospects for players in the global checkpoint inhibitor market. For instance, with the increase in awareness among people, the demand for these inhibitors is also increasing at a rapid pace globally.
Stringent Government Regulations to Restrain the Market Growth
Globally, several laws have been introduced to monitor the quality as well as the effectiveness of inhibitors. The regulatory requirements are becoming increasingly stringent, as biologics progress through clinical trials and to commercialization. Due to the nature of biologics, which are produced by living cells, the process and the product must be validated as well as strictly monitored against critical quality attributes (CQAs), throughout the manufacturing process, not just at the end as for chemistry-based drugs. This creates hurdles to getting regulatory approval for the biologics, which in turn is expected to impede the growth of the market.
Further, cancer treatment can cause many side effects, such as depression, tiredness, and trouble eating. The National Cancer Institute and the American Cancer Society describe many of these problems and offer tips for preventing or coping with them. Several side effects can happen months or years after treatment. Hence, the risk of complications associated with the treatment is another factor that will hamper the market growth.
High Cost of Inhibitors to Limit the Market Growth
Cancer treatment costs create significant economic challenges for healthcare providers as well as patients, because of the impact they have on patient safety, quality of care, and overall healthcare. The cost of cancer treatment is high, mainly due to laboratory tests, hospitalization, and the administration of novel checkpoint inhibitors (PD-1 and PD-L1) inhibitor therapeutics. The treatment cost of these therapeutics depends on the stage of cancer. In addition, the high cost is leading to a rise in the preference for alternative treatment options such as surgeries.
For instance, on average, the cost of cancer treatment in India is around INR 503,118 (USD 6,300) with a minimum expense of INR 90,561 (USD 1,134) and a maximum cost of INR 27,67,149 (USD 34,650). Also, the cost of available checkpoint inhibitors is high. Thus, the high cost associated with cancer treatment is a major factor that will restrain the treatment adoption rate especially in developing regions.
Favorable Initiatives by Regulatory Authorities in the Region to Fuel the Market Growth
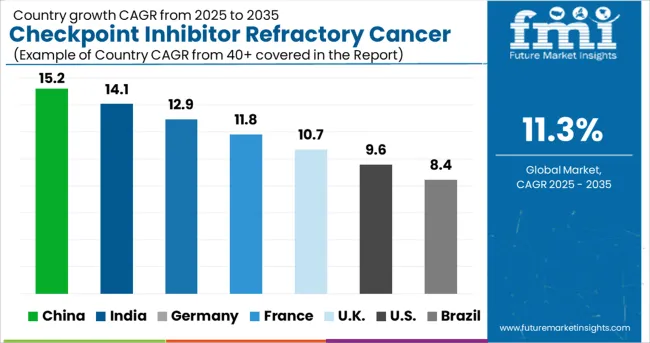
The Checkpoint Inhibitor Refractory Cancer Market in North America is expected to accumulate the highest market share of 39% in 2025.
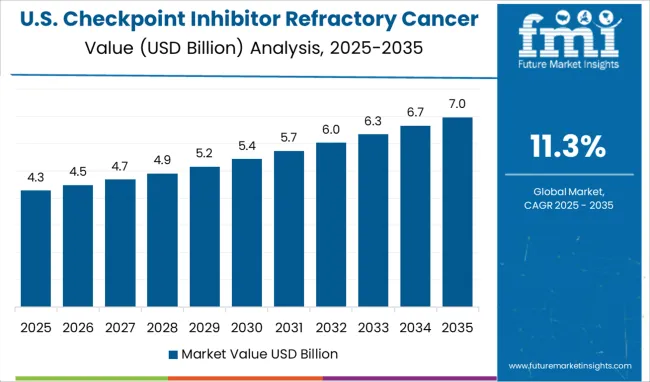
Factors such as favorable initiatives by the regulatory authorities in the region, especially in the USA, coupled with the increasing prevalence of cancer are expected to boost the growth of the checkpoint inhibitor refractory cancer market in North America. For instance, these inhibitors are used to treat a variety of cancer. As per the American Cancer Society, in 2025, there will be an estimated 1.9 million new cancer cases diagnosed and 609,360 cancer deaths in the USA. This in turn is anticipated to augment the market’s revenue share in the region.
The USA is estimated to account for around 99.2% of the North America Checkpoint inhibitors market in 2025. The significant share in the US is mainly attributed to the rising incidence of several chronic diseases such as urothelial carcinomas, lung cancers, skin cancer, and diabetes in the region, which demand efficient treatment.
For instance, according to a report from the American Cancer Society (2024), lung cancer is the second leading cancer resulting in 135,720 deaths in men as well as women. The numbers also report that around 84% of all lung cancers are NSCLC (non-small cell lung cancer) and 13% (small cell lung cancer) in the USA, which is expected to continue in the forthcoming years, leading to greater demand for checkpoint Inhibitors thus, fueling the market in the region. The region is expected to hold the highest CAGR of 11.1% during the forecast period.
Increasing Government Engagement to Surge Cancer Diagnosis in the Region to Fuel the Market Growth
The Checkpoint Inhibitor Refractory Cancer Market in Asia Pacific is expected to accumulate the highest market share of 37% in 2025.
The regional growth is attributed to the factors such as the high incidence of cancer, growing government engagement to surge cancer diagnosis, and growing adoption of inorganic growth strategies like collaborations, acquisitions, and mergers by major players functioning in the market introduction of new immunotherapies with lesser side effects, and increasing geriatric population in this region, especially in India. For instance, as per the cancer report by the Indian Council of Medical Research (ICMR), India’s cancer cases could increase by 12% in the next five years, with 47.9 million people projected to suffer from the non-communicable disease by 2025, up from 43 million in 2024. This is a major factor that will stimulate market growth.
Besides, China is expected to hold a significant growth rate over the analysis period, attributed to factors including a large population base, and major investment from private and public sectors into healthcare infrastructure development. Also, the growing interest in immune checkpoint inhibitors, coupled with the increase in the number of cancer cases, is anticipated to aid the development of the regional market in the near future. The region is expected to hold the highest CAGR of 11.0% during the forecast period.
Non-Small Cell Lung Segment to beat Competition in Untiring Market
On the basis of type, the global Checkpoint Inhibitor Refractory Cancer market is dominated by the Non-Small Cell Lung Segment, which accounts for a share of 37%. The segment is expected to hold a CAGR of 11.1% over the analysis period.
This significant share of non-small cell lung cancer has encouraged the majority of major players to concentrate on continuous innovation of advanced therapeutics and improving the efficacy of the present therapeutics.
Additionally, a growing number of diagnoses of NSCLC (non-small cell lung cancer), and the rising awareness in the nations of the developing and developed markets are supporting the growth of the non-small cell lung cancer segment.
Hospital Pharmacies Segment to Drive the Checkpoint Inhibitor Refractory Cancer Market
Based on the End-User, the hospital pharmacies segment is expected to witness a significant growth of 48.3% in 2025, and the trend is expected to continue during the forecast period, expanding at a rapid rate of 11.0% CAGR over the analysis period.
The segment’s growth is attributed to the factors such as large-scale orders, robust supply chains as well as attempts by hospital facilities to sustain large-scale inventories for cancer therapy applications. Moreover, the presence of advanced equipment and the availability of well-developed infrastructure adds to the development of the segment.
Further, the growing patient admission to hospitals for cancer treatment is surging the use of checkpoint inhibitors in hospitals, which in turn is anticipated to accelerate the sales in hospital pharmacies.
Checkpoint Inhibitor Refractory Cancer Market start-up players are adopting various marketing strategies such as new product launches, geographical expansion, mergers and acquisitions, partnerships, and collaboration to identify the interest of potential patients and create a larger customer base. For instance,
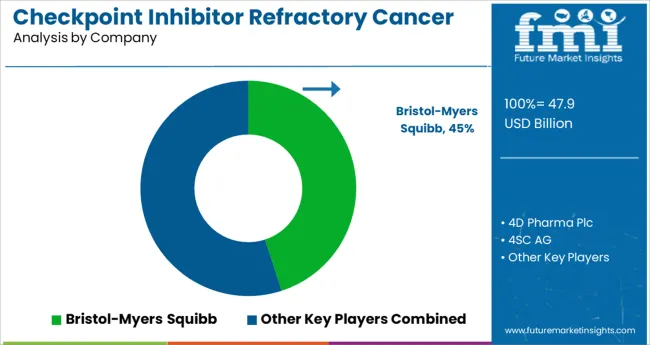
Prominent players in the Checkpoint Inhibitor Refractory Cancer market are Bristol-Myers Squibb, AstraZeneca, Merck, Genentech/Hoffmann-La Roche, Regeneron Pharmaceuticals, Merck KGaA, Pfizer, Bristol-Myers Squibb, Janssen Research and Development, LLC., 4D pharma plc., 4SC AG, OncoSec Medical, Mirati Therapeutics, Ascentage Pharma Group, and ENB Therapeutics, Inc., among others.
Recent Developments:
| Report Attribute | Details |
|---|---|
| Growth Rate | CAGR of 11.26% from 2025 to 2035 |
| Market Value in 2025 | USD 47.9 billion |
| Market Value in 2035 | USD 139.2 billion |
| Base Year for Estimation | 2025 |
| Historical Data | 2020 to 2025 |
| Forecast Period | 2025 to 2035 |
| Quantitative Units | Revenue in billion and CAGR from 2025 to 2035 |
| Report Coverage | Revenue Forecast, Company Ranking, Competitive Landscape, Growth Factors, Trends, and Pricing Analysis |
| Segments Covered | Type, Application, End-User, Region |
| Regions Covered | North America; Europe; Asia Pacific; Rest of the World |
| Key Countries Profiled | USA, Canada, Brazil, Mexico, Germany, UK, France, Spain, Italy, China, Japan, South Korea, Malaysia, Singapore, Australia, New Zealand, GCC Countries, South Africa, Israel |
| Key Companies Profiled | Bristol-Myers Squibb; AstraZeneca; Merck; Genentech/Hoffmann-La Roche; Regeneron Pharmaceuticals; Merck KGaA and Pfizer; Bristol-Myers Squibb; Janssen Research and Development, LLC; 4D pharma plc.; 4SC AG; OncoSec Medical; Mirati Therapeutics; Ascentage Pharma Group; ENB Therapeutics, Inc.; Exicure, Inc.; Evelo Biosciences, Inc./Merck Sharp & ; Dohme Corp.; Eisai; Kartos Therapeutics; Exelixis; ImmunityBio |
The global checkpoint inhibitor refractory cancer market is estimated to be valued at USD 47.9 billion in 2025.
It is projected to reach USD 139.2 billion by 2035.
The market is expected to grow at a 11.3% CAGR between 2025 and 2035.
The key product types are pd-1 inhibitor, ctla-4 inhibitor, pd-l1 inhibitor and others.
lung cancer segment is expected to dominate with a 38.0% industry share in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Immune Checkpoint Inhibitors Market
FcRn Inhibitors Market Size and Share Forecast Outlook 2025 to 2035
PARP Inhibitor Market Analysis – Size, Share, and Forecast Outlook 2025 to 2035
CGRP Inhibitors Market Trends - Growth, Demand & Forecast 2025 to 2035
Global KRAS Inhibitor Market Analysis – Size, Share & Forecast 2024-2034
PCSK9 Inhibitor Market Forecast Outlook 2025 to 2035
SGLT2 Inhibitors Market Size and Share Forecast Outlook 2025 to 2035
SGLT2 Inhibitors Treatment Market Overview – Trends & Growth 2024-2034
NF-KB Inhibitors Market
Mould Inhibitors Market
Kinase Inhibitor in Autoimmune Diseases Market Size and Share Forecast Outlook 2025 to 2035
Enzyme Inhibitors Market
Kinase Inhibitors For Cancer Treatment Market Size and Share Forecast Outlook 2025 to 2035
Galectin Inhibitor Therapeutics Market
Paraffin Inhibitors Market
Corrosion Inhibitors Market Growth - Trends & Forecast 2025 to 2035
PD-1/PD-L1 Inhibitors Market – Trends, Growth & Forecast 2025 to 2035
Mouse RNase Inhibitor Market Size and Share Forecast Outlook 2025 to 2035
Proton Pump Inhibitors Market Insights - Demand, Size & Industry Trends 2025 to 2035
Angiopoietin Inhibitors Therapeutic Market

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA