The global cardiogenic shock treatment market is expected to reach a market value of USD 1.03 billion in 2025 and USD 2.03 billion in 2035, reflecting a compound annual growth rate (CAGR) of 7% during the forecast period from 2025 to 2035.
In 2024, the Cardiogenic Shock Treatment industry registered growth at a steady pace due to the innovation in mechanical circulatory support devices and the growing use of ECMO (extracorporeal membrane oxygenation) in critical care. Top pharma companies introduced enhanced inotropic agents and vasopressors, which improved patient outcomes.
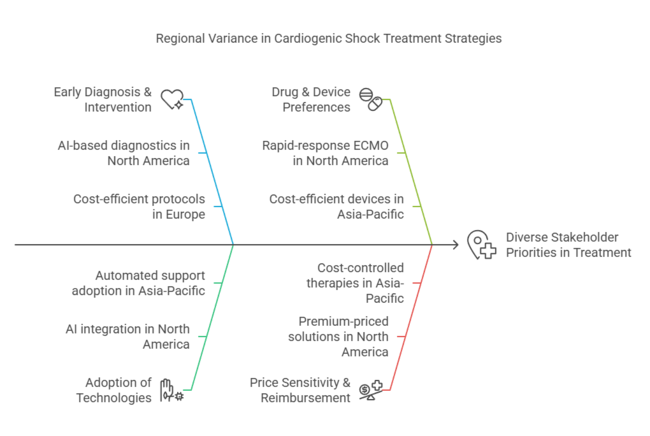
Launch approvals for new drug-device combinations, especially in Europe and North America, also contributed to the growth of the industry. In addition, greater awareness and earlier diagnosis campaigns resulted in greater treatment adoption rates, particularly among high-risk patient groups, including those with acute myocardial infarction (AMI) and advanced heart failure.
Despite this advancement, supply chain interruptions led to sporadic shortages of essential drugs, and hospitals were forced to seek alternative treatment protocols. The Asia-Pacific region witnessed heavy investments in cardiac critical care facilities, with nations such as China and India increasing specialized heart centers.
The industry is anticipated to witness innovation in minimally invasive hemodynamic smart technologies by 2035. AI-based predictive analytics for early detection of cardiogenic shock is also picking up steam. Industry growth will be supported by increasing healthcare access in developing economies, while ongoing clinical trials for next-generation vasodilators and gene therapies have the potential to reshape treatment paradigms in the decade ahead.
| Metrics | Values |
|---|---|
| Industry size (2025E) | USD 1.03 billion |
| Industry Value (2035F) | USD 2.03 billion |
| CAGR (2025 to 2035) | 7% |
The Cardiogenic Shock Treatment industry is growing strongly, fueled by technology in mechanical circulatory support, AI-assisted diagnostics, and increasing access to critical care within emerging economies. Top pharma and med-tech firms have the potential to gain from increasing demand, while hospitals with outmoded infrastructure face being left behind. Strategic investment in innovation and cross-country partnerships will make or break long-term leadership.
Speed Up Innovation in Minimally Invasive Technologies
Executives need to focus on R&D in next-generation mechanical circulatory support devices and AI-based early detection solutions to enhance survival rates and lower ICU burden.
Enhance Industry Penetration in Emerging Economies
Harmonize product development and pricing strategies with the expanding healthcare infrastructure in Asia-Pacific and Latin America to capture unmet demand and broaden the global reach.
Form Strategic Alliances & M&A for Competitive Advantage
Seek acquisitions or mergers with biotech companies, health tech companies with AI capabilities, and hospital groups to deepen product offerings, rationalize distribution, and achieve regulatory approvals in top industries.
| Risk | Probability & Impact |
|---|---|
| Regulatory Delays for New Treatments | High - Could be a slow industry entry for innovative therapies, affecting revenue growth. |
| Supply Chain Disruptions | Medium - May lead to shortages of critical drugs and devices, increasing treatment costs. |
| Market Consolidation & Competitive Pressure | Medium - Mergers and acquisitions can create dominant players, making it more challenging for smaller firms to compete. |
| Priority | Immediate Action |
|---|---|
| Expand R&D for AI-Driven Early Detection | Fund pilot programs integrating AI diagnostics into ICU workflows. |
| Secure Emerging Industry Distribution | Establish regional partnerships to navigate local regulatory and reimbursement challenges. |
| Optimize Supply Chain Resilience | Diversify sourcing and invest in predictive inventory management systems. |
Cardiogenic Shock Treatment industry leaders must, therefore, step up innovations in minimally-invasive support technologies, strengthen the global distribution network, and ensure greater supply chain resilience to retain industry leadership in troubling times.
As patient care model re-invention follows on the heels of AI-powered diagnostic support, investing up-front in data-linked treatment opportunities will be imperative. The next 12 months should be focused on accelerating regulatory approvals, forming strategic partnerships in high-growth industries and scaling production capacity to meet growing demand.
| Countries/Region | Regulatory Impact & Mandatory Certifications |
|---|---|
| United States | FDA Approval (510(k), PMA, or Breakthrough Device Program) - Strict regulatory oversight by the FDA affects the speed of new drug and device approvals. The CMS reimbursement policies also influence hospital adoption rates for advanced treatments. |
| European Union | EU MDR (Medical Device Regulation 2017/745) - Calls for stringent clinical evidence for device approvals, raising R&D expenses. EMA (European Medicines Agency) regulation impacts drug approvals, prioritizing safety and cost-effectiveness. Reimbursement guidelines are country-specific but, in general, tend to support evidence-based, cost-effective therapies. |
| United Kingdom | MHRA (Medicines and Healthcare products Regulatory Agency) Certification - Following Brexit, businesses are required to meet the UK's CA (UK Conformity Assessed) marking in place of the EU CE mark. The NHS procurement framework has a preference for cost-saving treatments, which influences the uptake of expensive innovations. |
| China | NMPA (National Medical Products Administration) Approval - Mandates local clinical trials for imported drugs and devices, slowing industry entry. Government price controls on medical devices restrict sales of premium products, benefiting local manufacturers. |
| Japan | PMDA (Pharmaceuticals and Medical Devices Agency) Approval - High post-industry surveillance standards raise compliance expenses. Reimbursement prices are determined by the Central Social Insurance Medical Council (Chuikyo), which can limit pricing flexibility for expensive treatments. |
| India | CDSCO (Central Drugs Standard Control Organization) & Medical Devices Rules 2017 - Demands specific registration for imported cardiovascular devices. The government's Ayushman Bharat healthcare program is driving the demand for affordable solutions, capping the industry for high-end therapies. |
| South Korea | MFDS (Ministry of Food and Drug Safety) Certification - Demands local approval for high-risk devices. Reimbursement policies prefer low-cost options, capping the speedy adoption of advanced treatments. |
| Brazil | ANVISA (Agência Nacional de Vigilância Sanitária) Certification - Long approval times for new medicines and equipment. Public healthcare system (SUS) budget limitations affect industry access to costly treatments. |
| Australia | TGA (Therapeutic Goods Administration) Registration - Needs compliance with international regulatory requirements (FDA/EU MDR), yet approvals remain time-consuming. Medicare reimbursement policies drive hospital uptake rates for innovative cardiogenic shock treatments. |
The United States will have a marginally higher than the overall average growth by the following decade, attributable to its world-class healthcare system and robust R&D ecosystem. The Breakthrough Device Program initiated by the FDA is streamlining approvals for revolutionary therapies such as next-generation mechanical circulatory assist devices like ECMO and pVADs. Growing AI implementation in early shock detection is improving patient results, further fueling industry growth.
Moreover, Medicare and Medicaid reimbursement policies also have a vital role in treatment acceptance, especially in cases of high-value therapies. Supply chain constraints and increasing healthcare expenditures may, however, slow down the pace. Industry leaders such as Abbott and Medtronic are still making R&D investments, keeping the USA at the forefront of cardiogenic shock therapy.
FMI opines that the United States cardiogenic shock treatment sales will grow at nearly 7.8% CAGR through 2025 to 2035.
The UK industry is projected to improve as a result of NHS budget limitations and changes in regulations following Brexit. Post-Brexit, the MHRA (Medicines and Healthcare Products Regulatory Agency) now requires UKCA marking for medical devices, increasing the complexity of product approvals. Despite this, investments in AI-based diagnostics and ECMO therapy growth are fueling demand.
The UK's National Health Service (NHS) focuses on cost-saving measures, with treatments that minimize ICU stays and long-term care expenses being preferred. Government support for cardiovascular research, especially by the British Heart Foundation, drives industry growth. In spite of regulatory challenges, the presence of large medical device manufacturers guarantees ongoing industry growth, with London becoming a central hub for cardiovascular innovation.
FMI opines that the United Kingdom cardiogenic shock treatment sales will grow at nearly 6.5% CAGR through 2025 to 2035.
France is also expected to grow with its superior healthcare infrastructure and government backing for cardiovascular research. The Haute Autorité de Santé (HAS) considers the cost-effectiveness of emerging therapies before allowing reimbursement, with industry access proving difficult for high-priced devices. The increasing uptake of AI-aided diagnostics and minimally invasive treatments like catheter-based therapy is driving demand, however.
France's Plan Santé 2030 is designed to improve critical care centers, such as enhanced cardiac ICUs, that continue to drive industry opportunities. Domestic companies, like Getinge and LivaNova, are targeting novel mechanical circulatory support devices, maintaining France's position as an influential force in the European cardiogenic shock treatment industry.
FMI opines that France's cardiogenic shock treatment sales will grow at nearly 6.8% CAGR through 2025 to 2035.
Germany is projected to expand due to its sophisticated medical infrastructure and robust reimbursement policies. Industry access is regulated by the Federal Joint Committee (G-BA), which requires new therapies to pass rigorous cost-benefit analyses prior to approval. Germany is a leader in ECMO uptake, with more than 250 cardiac centers having incorporated sophisticated circulatory support systems.
The nation also enjoys one of the highest rates of adoption of AI-based early shock detection, increasing patient survival levels. Innovation in the management of cardiogenic shock is supported with government funding under the German Research Foundation (DFG). An escalation of the regulatory compliance expenditure under the EU MDR (Medical Device Regulation) may provide challenges to entry for new companies, though.
FMI opines that the Germany cardiogenic shock treatment sales will grow at nearly 7.2% CAGR through 2025 to 2035.
Italy's cardiogenic shock treatment industry is projected to expand slightly due to its economic limitations and regional differences in healthcare facilities. The Agenzia Italiana del Farmaco (AIFA) regulates drug and device approvals, usually leading to long regulatory horizons. But advances in telemedicine and AI-based cardiology solutions are enhancing early detection rates. Italy's elderly population is raising the incidence of heart failure, fueling demand for mechanical circulatory support and inotropic therapy.
The National Health System (SSN) is investing in specialized cardiac ICUs, especially in northern Italy, but budget limitations could constrain wider industry expansion. Collaborations between international medical device companies and Italian hospitals are assisting in the modernization of treatment strategies.
FMI opines that the Italy cardiogenic shock treatment sales will grow at nearly 6.6% CAGR through 2025 to 2035.
South Korea's growth is driven by large government investments in digital healthcare and medical technology. Cardiogenic shock treatments are regulated by the Ministry of Food and Drug Safety (MFDS), and the fast-track authorization process is paving the way for industry growth. South Korea's robotic-assisted cardiac care lead is evident from Seoul hospitals integrating AI-powered early detection systems into their practices.
The government’s Health Insurance Review and Assessment Service (HIRA) ensures broad access to advanced treatments, including ECMO and pVADs. However, high import taxes on medical devices increase costs for hospitals. Domestic firms such as Mediplex and Sejong Medical are gaining industry share, focusing on cost-effective mechanical circulatory support solutions.
FMI opines that the South Korean cardiogenic shock treatment sales will grow at nearly 7.5% CAGR through 2025 to 2035.
Japan's cardiogenic shock treatment industry is expected to expand slightly due to a preference for affordable, long-term treatment options. The Pharmaceuticals and Medical Devices Agency (PMDA) has rigorous approval procedures, which makes it difficult for foreign companies to enter the industry in a short time.
Japan's Central Social Insurance Medical Council (Chuikyo) determines reimbursement prices, focusing on affordability over expensive innovations. The demographic bulge is boosting long-term cardiac care demand, prompting the use of minimally invasive procedures. Terumo and Nipro are among the companies leading local innovation in circulatory support devices.
FMI opines that the Japan cardiogenic shock treatment sales will grow at nearly 6.4% CAGR through 2025 to 2035.
China’s cardiogenic shock treatment industry is fuelled by quick healthcare infrastructure growth and state investment in emergency care. China's National Medical Products Administration has speeded up the approval rate for life-support cardiovascular devices, with both Chinese and foreign suppliers reaping rewards.
China is making significant investments in ECMO and AI-assisted early diagnostic devices, and top hospitals in Beijing and Shanghai are driving adoption. However, government price regulation on medical devices caps the profitability of high-end therapies.
FMI opines that China’s cardiogenic shock treatment sales will grow at nearly 8.2% CAGR through 2025 to 2035.
Australia and New Zealand's cardiogenic shock treatment industry will grow slightly because of stringent regulatory requirements and cost-containment measures. Australia's Therapeutic Goods Administration (TGA) and New Zealand's Medsafe demand robust clinical trial information before approving new treatments, delaying industry entry.
However, both nations are early adopters of AI-based cardiac diagnosis and telemedicine-based cardiology consultations, which enhance early detection rates. Cost-saving treatment is encouraged by reimbursement policies under Medicare (Australia) and Pharmac (New Zealand), making it difficult for high-end medical device makers to pick up sales. Meanwhile, collaborations between public hospitals and multinational healthcare companies are broadening access to advanced circulatory support systems, especially in Sydney, Melbourne, and Auckland.
FMI opines that the Australia-NZ cardiogenic shock treatment sales will grow at nearly 6.7% CAGR through 2025 to 2035.
The cardiogenic shock treatment industry is experiencing ongoing development, with several new in-vitro test kits for diagnosis and drugs and devices for treatment. The adoption of in-vitro test kits is rapidly increasing because timely diagnosis ensures higher survival rates by allowing for targeted and timely intervention. Biomarker-based test kits investment by healthcare facilities for diagnostic accuracy. The additional pharmaceutical segment is expanding gradually, with continuing research and improvement routine on vasoactive prescription drugs and new helpful agents to solidifying hemodynamics.
Drug-based treatment is still the front-line therapy approach, even as approved cardiovascular medications expand their indications, lending credence to industry growth. Devices like ECMO and percutaneous ventricular assist devices (pVADs) have become standards of care in such settings, with sustained improvements in miniaturization and efficiency. The technical evolution of these entities, all combined with a positive regulation route, are driving their global cornerstone.
In addition, more investments in AI-driven monitoring tools are constantly improving device performance and patient outcomes. The sector is likely to undertake growth at an average CAGR of 7.2% by 2035.
End-user dynamics are evolving as advanced cardiogenic shock treatments are integrated into the routine normal care of hospitals, cardiac catheterisation labs and ambulatory surgical centers. The highest level of adoption is seen in hospitals, owing to their established specialized critical care units and ECMO centers.
Acute drug use is a unique challenge that drives the utilization of hospital-based drug and general programs. More recently, cardiac catheterization labs have scaled their capacities from not only diagnostic procedures but also interventional therapies, including mechanical circulatory support and percutaneous coronary interventions.
They are providing quicker and less invasive treatments for cardiogenic shock, which improve patient survival and outcomes. Ambulatory surgical centers are emerging as lower-cost alternatives, particularly in developed industries that are seeing increased outpatient cardiovascular procedures.
These centers are also utilizing AI-based monitoring tools and telemedicine consultations to maximize post-procedure care, which is likely to contribute to industry growth within a value-driven healthcare environment. The segment will expand at a CAGR of 6.8% throughout the forecast period between 2025 and 2035.
The treatment industry for cardiogenic shock is fairly concentrated, with major players such as Abbott Laboratories, Abiomed, and Medtronic controlling substantial industry shares. They compete based on innovation, strategic alliances, and geographical expansion. Avenacy introduced a generic equivalent for cardiogenic shock treatment, increasing industry competition, in May 2024.
In January 2024, LivaNova PLC revealed intentions to wind down its Advanced Circulatory Support Business Unit by the end of 2024, with the intention of concentrating on core areas. Also, in October 2024, Abbott began the TEAM-HF clinical trial to enhance heart failure patients' outcomes, which could have an effect on future treatment strategies.
Abiomed, now a part of Johnson & Johnson, commands approximately 45-50% of the temporary mechanical circulatory support market as of 2025. Its dominance is anchored by the Impella heart pump platform, which remains the preferred choice for short-term cardiac support in high-risk percutaneous coronary interventions and cardiogenic shock. Abiomed's early mover advantage, combined with continuous innovation and aggressive physician education programs, has made it difficult for competitors to meaningfully erode its lead.
Getinge AB follows with an estimated 18-20% market share, although it is experiencing a slow decline. The company’s traditional technologies are facing increasing pressure from next-generation devices offering superior ease of use and patient outcomes. As a result, Getinge’s relative standing is expected to diminish unless it accelerates innovation or pivots its portfolio.
LivaNova holds between 12-15% of the market, largely through its extracorporeal membrane oxygenation (ECMO) systems designed for patients with severe cardiogenic shock. While not a direct substitute for short-term heart pumps like Impella, LivaNova’s ECMO offerings serve adjacent clinical needs, allowing the company to maintain a solid presence in critical care environments.
Medtronic controls around 8-10% of the market, leveraging its specialized heart-support devices and proprietary guide wires targeted at cardiogenic shock therapy. Although Medtronic remains a formidable healthcare company overall, in this niche it has yet to create a disruptive product that could challenge Abiomed’s dominance.
Terumo Corporation holds roughly 5-7% of the market with its cardiovascular support products. While respected for quality, Terumo’s offerings are often seen as complementary rather than competitive to the flagship devices of larger players.
The remaining 8-10% of the market is distributed among smaller players such as Teleflex, Edwards Lifesciences, and a group of startups pushing into the space with emerging technologies. Although their footprint remains limited for now, these companies are developing next-generation mechanical circulatory support systems that could, over the next five to seven years, materially alter the competitive landscape if clinical outcomes prove superior.
Cardiogenic shock treatment aims to stabilize blood flow and improve heart function, often using medications, devices, and mechanical circulatory support.
Common therapies include vasoactive drugs, inotropes, ECMO devices, and percutaneous ventricular assist devices (pVADs).
Hospitals, cardiac catheterization labs, and ambulatory surgical centers are the primary users of these treatments.
It is diagnosed using blood tests, imaging, and in-vitro test kits to assess heart function and circulation.
The rising prevalence of heart diseases, advancements in treatment technologies, and improved diagnostic methods are key drivers.
Table 1: Global Market Value (US$ Million) Forecast by Region, 2018 to 2033
Table 2: Global Market Value (US$ Million) Forecast by Treatment Type, 2018 to 2033
Table 3: Global Market Value (US$ Million) Forecast by End-User, 2018 to 2033
Table 4: North America Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 5: North America Market Value (US$ Million) Forecast by Treatment Type, 2018 to 2033
Table 6: North America Market Value (US$ Million) Forecast by End-User, 2018 to 2033
Table 7: Latin America Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 8: Latin America Market Value (US$ Million) Forecast by Treatment Type, 2018 to 2033
Table 9: Latin America Market Value (US$ Million) Forecast by End-User, 2018 to 2033
Table 10: Europe Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 11: Europe Market Value (US$ Million) Forecast by Treatment Type, 2018 to 2033
Table 12: Europe Market Value (US$ Million) Forecast by End-User, 2018 to 2033
Table 13: South Asia Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 14: South Asia Market Value (US$ Million) Forecast by Treatment Type, 2018 to 2033
Table 15: South Asia Market Value (US$ Million) Forecast by End-User, 2018 to 2033
Table 16: East Asia Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 17: East Asia Market Value (US$ Million) Forecast by Treatment Type, 2018 to 2033
Table 18: East Asia Market Value (US$ Million) Forecast by End-User, 2018 to 2033
Table 19: Oceania Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 20: Oceania Market Value (US$ Million) Forecast by Treatment Type, 2018 to 2033
Table 21: Oceania Market Value (US$ Million) Forecast by End-User, 2018 to 2033
Table 22: MEA Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 23: MEA Market Value (US$ Million) Forecast by Treatment Type, 2018 to 2033
Table 24: MEA Market Value (US$ Million) Forecast by End-User, 2018 to 2033
Figure 1: Global Market Value (US$ Million) by Treatment Type, 2022 to 2033
Figure 2: Global Market Value (US$ Million) by End-User, 2022 to 2033
Figure 3: Global Market Value (US$ Million) by Region, 2022 to 2033
Figure 4: Global Market Value (US$ Million) Analysis by Region, 2018-2033
Figure 5: Global Market Value Share (%) and BPS Analysis by Region, 2022 to 2033
Figure 6: Global Market Y-o-Y Growth (%) Projections by Region, 2022 to 2033
Figure 7: Global Market Value (US$ Million) Analysis by Treatment Type, 2018-2033
Figure 8: Global Market Value Share (%) and BPS Analysis by Treatment Type, 2022 to 2033
Figure 9: Global Market Y-o-Y Growth (%) Projections by Treatment Type, 2022 to 2033
Figure 10: Global Market Value (US$ Million) Analysis by End-User, 2018-2033
Figure 11: Global Market Value Share (%) and BPS Analysis by End-User, 2022 to 2033
Figure 12: Global Market Y-o-Y Growth (%) Projections by End-User, 2022 to 2033
Figure 13: Global Market Attractiveness by Treatment Type, 2022 to 2033
Figure 14: Global Market Attractiveness by End-User, 2022 to 2033
Figure 15: Global Market Attractiveness by Region, 2022 to 2033
Figure 16: North America Market Value (US$ Million) by Treatment Type, 2022 to 2033
Figure 17: North America Market Value (US$ Million) by End-User, 2022 to 2033
Figure 18: North America Market Value (US$ Million) by Country, 2022 to 2033
Figure 19: North America Market Value (US$ Million) Analysis by Country, 2018-2033
Figure 20: North America Market Value Share (%) and BPS Analysis by Country, 2022 to 2033
Figure 21: North America Market Y-o-Y Growth (%) Projections by Country, 2022 to 2033
Figure 22: North America Market Value (US$ Million) Analysis by Treatment Type, 2018-2033
Figure 23: North America Market Value Share (%) and BPS Analysis by Treatment Type, 2022 to 2033
Figure 24: North America Market Y-o-Y Growth (%) Projections by Treatment Type, 2022 to 2033
Figure 25: North America Market Value (US$ Million) Analysis by End-User, 2018-2033
Figure 26: North America Market Value Share (%) and BPS Analysis by End-User, 2022 to 2033
Figure 27: North America Market Y-o-Y Growth (%) Projections by End-User, 2022 to 2033
Figure 28: North America Market Attractiveness by Treatment Type, 2022 to 2033
Figure 29: North America Market Attractiveness by End-User, 2022 to 2033
Figure 30: North America Market Attractiveness by Country, 2022 to 2033
Figure 31: Latin America Market Value (US$ Million) by Treatment Type, 2022 to 2033
Figure 32: Latin America Market Value (US$ Million) by End-User, 2022 to 2033
Figure 33: Latin America Market Value (US$ Million) by Country, 2022 to 2033
Figure 34: Latin America Market Value (US$ Million) Analysis by Country, 2018-2033
Figure 35: Latin America Market Value Share (%) and BPS Analysis by Country, 2022 to 2033
Figure 36: Latin America Market Y-o-Y Growth (%) Projections by Country, 2022 to 2033
Figure 37: Latin America Market Value (US$ Million) Analysis by Treatment Type, 2018-2033
Figure 38: Latin America Market Value Share (%) and BPS Analysis by Treatment Type, 2022 to 2033
Figure 39: Latin America Market Y-o-Y Growth (%) Projections by Treatment Type, 2022 to 2033
Figure 40: Latin America Market Value (US$ Million) Analysis by End-User, 2018-2033
Figure 41: Latin America Market Value Share (%) and BPS Analysis by End-User, 2022 to 2033
Figure 42: Latin America Market Y-o-Y Growth (%) Projections by End-User, 2022 to 2033
Figure 43: Latin America Market Attractiveness by Treatment Type, 2022 to 2033
Figure 44: Latin America Market Attractiveness by End-User, 2022 to 2033
Figure 45: Latin America Market Attractiveness by Country, 2022 to 2033
Figure 46: Europe Market Value (US$ Million) by Treatment Type, 2022 to 2033
Figure 47: Europe Market Value (US$ Million) by End-User, 2022 to 2033
Figure 48: Europe Market Value (US$ Million) by Country, 2022 to 2033
Figure 49: Europe Market Value (US$ Million) Analysis by Country, 2018-2033
Figure 50: Europe Market Value Share (%) and BPS Analysis by Country, 2022 to 2033
Figure 51: Europe Market Y-o-Y Growth (%) Projections by Country, 2022 to 2033
Figure 52: Europe Market Value (US$ Million) Analysis by Treatment Type, 2018-2033
Figure 53: Europe Market Value Share (%) and BPS Analysis by Treatment Type, 2022 to 2033
Figure 54: Europe Market Y-o-Y Growth (%) Projections by Treatment Type, 2022 to 2033
Figure 55: Europe Market Value (US$ Million) Analysis by End-User, 2018-2033
Figure 56: Europe Market Value Share (%) and BPS Analysis by End-User, 2022 to 2033
Figure 57: Europe Market Y-o-Y Growth (%) Projections by End-User, 2022 to 2033
Figure 58: Europe Market Attractiveness by Treatment Type, 2022 to 2033
Figure 59: Europe Market Attractiveness by End-User, 2022 to 2033
Figure 60: Europe Market Attractiveness by Country, 2022 to 2033
Figure 61: South Asia Market Value (US$ Million) by Treatment Type, 2022 to 2033
Figure 62: South Asia Market Value (US$ Million) by End-User, 2022 to 2033
Figure 63: South Asia Market Value (US$ Million) by Country, 2022 to 2033
Figure 64: South Asia Market Value (US$ Million) Analysis by Country, 2018-2033
Figure 65: South Asia Market Value Share (%) and BPS Analysis by Country, 2022 to 2033
Figure 66: South Asia Market Y-o-Y Growth (%) Projections by Country, 2022 to 2033
Figure 67: South Asia Market Value (US$ Million) Analysis by Treatment Type, 2018-2033
Figure 68: South Asia Market Value Share (%) and BPS Analysis by Treatment Type, 2022 to 2033
Figure 69: South Asia Market Y-o-Y Growth (%) Projections by Treatment Type, 2022 to 2033
Figure 70: South Asia Market Value (US$ Million) Analysis by End-User, 2018-2033
Figure 71: South Asia Market Value Share (%) and BPS Analysis by End-User, 2022 to 2033
Figure 72: South Asia Market Y-o-Y Growth (%) Projections by End-User, 2022 to 2033
Figure 73: South Asia Market Attractiveness by Treatment Type, 2022 to 2033
Figure 74: South Asia Market Attractiveness by End-User, 2022 to 2033
Figure 75: South Asia Market Attractiveness by Country, 2022 to 2033
Figure 76: East Asia Market Value (US$ Million) by Treatment Type, 2022 to 2033
Figure 77: East Asia Market Value (US$ Million) by End-User, 2022 to 2033
Figure 78: East Asia Market Value (US$ Million) by Country, 2022 to 2033
Figure 79: East Asia Market Value (US$ Million) Analysis by Country, 2018-2033
Figure 80: East Asia Market Value Share (%) and BPS Analysis by Country, 2022 to 2033
Figure 81: East Asia Market Y-o-Y Growth (%) Projections by Country, 2022 to 2033
Figure 82: East Asia Market Value (US$ Million) Analysis by Treatment Type, 2018-2033
Figure 83: East Asia Market Value Share (%) and BPS Analysis by Treatment Type, 2022 to 2033
Figure 84: East Asia Market Y-o-Y Growth (%) Projections by Treatment Type, 2022 to 2033
Figure 85: East Asia Market Value (US$ Million) Analysis by End-User, 2018-2033
Figure 86: East Asia Market Value Share (%) and BPS Analysis by End-User, 2022 to 2033
Figure 87: East Asia Market Y-o-Y Growth (%) Projections by End-User, 2022 to 2033
Figure 88: East Asia Market Attractiveness by Treatment Type, 2022 to 2033
Figure 89: East Asia Market Attractiveness by End-User, 2022 to 2033
Figure 90: East Asia Market Attractiveness by Country, 2022 to 2033
Figure 91: Oceania Market Value (US$ Million) by Treatment Type, 2022 to 2033
Figure 92: Oceania Market Value (US$ Million) by End-User, 2022 to 2033
Figure 93: Oceania Market Value (US$ Million) by Country, 2022 to 2033
Figure 94: Oceania Market Value (US$ Million) Analysis by Country, 2018-2033
Figure 95: Oceania Market Value Share (%) and BPS Analysis by Country, 2022 to 2033
Figure 96: Oceania Market Y-o-Y Growth (%) Projections by Country, 2022 to 2033
Figure 97: Oceania Market Value (US$ Million) Analysis by Treatment Type, 2018-2033
Figure 98: Oceania Market Value Share (%) and BPS Analysis by Treatment Type, 2022 to 2033
Figure 99: Oceania Market Y-o-Y Growth (%) Projections by Treatment Type, 2022 to 2033
Figure 100: Oceania Market Value (US$ Million) Analysis by End-User, 2018-2033
Figure 101: Oceania Market Value Share (%) and BPS Analysis by End-User, 2022 to 2033
Figure 102: Oceania Market Y-o-Y Growth (%) Projections by End-User, 2022 to 2033
Figure 103: Oceania Market Attractiveness by Treatment Type, 2022 to 2033
Figure 104: Oceania Market Attractiveness by End-User, 2022 to 2033
Figure 105: Oceania Market Attractiveness by Country, 2022 to 2033
Figure 106: MEA Market Value (US$ Million) by Treatment Type, 2022 to 2033
Figure 107: MEA Market Value (US$ Million) by End-User, 2022 to 2033
Figure 108: MEA Market Value (US$ Million) by Country, 2022 to 2033
Figure 109: MEA Market Value (US$ Million) Analysis by Country, 2018-2033
Figure 110: MEA Market Value Share (%) and BPS Analysis by Country, 2022 to 2033
Figure 111: MEA Market Y-o-Y Growth (%) Projections by Country, 2022 to 2033
Figure 112: MEA Market Value (US$ Million) Analysis by Treatment Type, 2018-2033
Figure 113: MEA Market Value Share (%) and BPS Analysis by Treatment Type, 2022 to 2033
Figure 114: MEA Market Y-o-Y Growth (%) Projections by Treatment Type, 2022 to 2033
Figure 115: MEA Market Value (US$ Million) Analysis by End-User, 2018-2033
Figure 116: MEA Market Value Share (%) and BPS Analysis by End-User, 2022 to 2033
Figure 117: MEA Market Y-o-Y Growth (%) Projections by End-User, 2022 to 2033
Figure 118: MEA Market Attractiveness by Treatment Type, 2022 to 2033
Figure 119: MEA Market Attractiveness by End-User, 2022 to 2033
Figure 120: MEA Market Attractiveness by Country, 2022 to 2033






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Shock Testing System Market Size and Share Forecast Outlook 2025 to 2035
Shock Tube System Market Size and Share Forecast Outlook 2025 to 2035
Shock Sensor Market Size and Share Forecast Outlook 2025 to 2035
Shock Data Logger Market
Shock Test Machines Market
Anti-shock Trousers Market
Motorcycle Shock Absorbers Market
High Energy Shockwave Therapy Units Market Size and Share Forecast Outlook 2025 to 2035
Erectile Dysfunction Shockwave Generators Market
Automotive Gas Charged Shock Absorbers Market Growth - Trends & Forecast 2025 to 2035
Treatment-Resistant Hypertension Management Market Size and Share Forecast Outlook 2025 to 2035
Treatment-Resistant Depression Treatment Market Size and Share Forecast Outlook 2025 to 2035
Treatment Pumps Market Insights Growth & Demand Forecast 2025 to 2035
Pretreatment Coatings Market Size and Share Forecast Outlook 2025 to 2035
Air Treatment Ozone Generator Market Size and Share Forecast Outlook 2025 to 2035
CNS Treatment and Therapy Market Insights - Trends & Growth Forecast 2025 to 2035
Seed Treatment Materials Market Size and Share Forecast Outlook 2025 to 2035
Acne Treatment Solutions Market Size and Share Forecast Outlook 2025 to 2035
Scar Treatment Market Overview - Growth & Demand Forecast 2025 to 2035
Soil Treatment Chemicals Market

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA