The global automated external defibrillator (AED) market is projected to reach USD 1.39 billion in 2025 and grow to USD 2.20 billion by 2035, reflecting a CAGR of 4.7% over the forecast period. This growth is primarily driven by increasing awareness of cardiac emergencies, the rising incidence of cardiovascular diseases, and advancements in AED technology that improve ease of use and accessibility.
AEDs are critical devices used to treat sudden cardiac arrest (SCA) by delivering a controlled electric shock to the heart, restoring its normal rhythm. As more public places, workplaces, and sporting venues install AEDs to increase the chances of survival in case of cardiac emergencies, the market for these devices is expanding rapidly. Governments and health organizations are also promoting the installation of AEDs in public spaces to improve emergency preparedness, contributing to market growth.
Recent innovations in AED technology have focused on making the devices more user-friendly and accessible. In 2025, Philips Healthcare introduced an advanced AED model equipped with real-time feedback for CPR quality, including chest compression depth and rate, to help lay responders provide more effective care.
Additionally, Zoll Medical Corporation launched the Zoll AED 3, which features a color touchscreen, enhanced ease of use, and real-time visual and audio prompts, making it suitable for use in high-stress situations by non-medical personnel.
The growing trend of personalized healthcare has also influenced the AED market, with companies increasingly offering portable, lightweight, and battery-efficient models. Furthermore, the integration of cloud-based data storage and smart connectivity into AED devices allows for remote monitoring, real-time diagnostics, and maintenance tracking, which enhance device reliability and support quicker response times in emergencies.
The market’s regional dynamics show that North America continues to dominate the AED market, driven by regulatory support and widespread adoption in public places. However, Asia-Pacific is expected to witness the fastest growth, fueled by increasing healthcare infrastructure investments and awareness campaigns in countries like China and India.
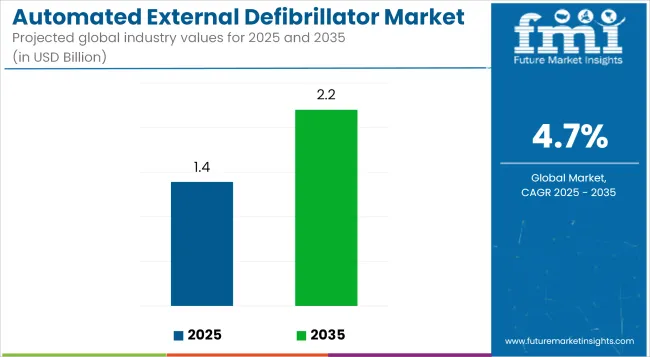
| Attributes | Description |
|---|---|
| Estimated Automated External Defibrillator Market Size (2025E) | USD 1.39 billion |
| Projected Automated External Defibrillator Market Value (2035F) | USD 2.20 billion |
| Value-based CAGR (2025 to 2035) | 4.70% |
The Automated External Defibrillator (AED) market is set for strong growth, with the fully automated AED segment expected to dominate the market due to its ease of use and efficiency. Public access sites are projected to hold the largest share of the market, driven by increasing awareness and efforts to enhance emergency medical response systems in public spaces.
The Fully Automated External Defibrillator (AED) segment is projected to capture 85.4% of the market share by 2025. Fully automated AEDs are favored for their ability to automatically analyze a patient’s heart rhythm and deliver a shock without requiring manual intervention, making them more user-friendly, especially in emergency situations where time is critical. These devices are increasingly deployed in public spaces, workplaces, and healthcare settings due to their ease of use, which minimizes the need for specialized medical training.
Key players like Philips Healthcare, Zoll Medical Corporation, and Stryker Corporation are leading the development of these devices, integrating advanced technology to improve performance, safety, and portability. The superior safety and efficiency of fully automated AEDs are driving their widespread adoption, ensuring their continued dominance in the AED market through 2025.
The Public Access segment is expected to dominate the Automated External Defibrillator (AED) market, holding 80.6% of the total market share by 2025. Public access sites, including schools, airports, shopping malls, and sports arenas, are key growth drivers for the AED market. As governments and organizations place increased emphasis on improving emergency response capabilities, the demand for AEDs in public spaces has surged. The widespread placement of AEDs in public areas ensures that life-saving equipment is accessible during cardiac emergencies.
Partnerships between AED manufacturers and public organizations, such as American Heart Association and Red Cross, are boosting market growth by promoting initiatives to place AEDs in public spaces. The high demand for rapid medical intervention, coupled with the increasing awareness of sudden cardiac arrest, is expected to fuel further growth of the public access AED market, ensuring its leadership in the market through 2025.
Emphasis of Manufacturers on Launch of New Products to the Market Surges the Market Growth
The evolving needs of healthcare facilities encourage manufacturers to prioritize the development and innovation of a user-friendly AED devices that has ability to cater broader range of medical settings which has an inclusion of hospitals, clinics, and public spaces. This demand encourages manufacturers to develop products that are lightweight and portable.
Moreover, in addition to stay ahead in the competition they are including several advanced features in their products such as wireless connectivity that also allows remote monitoring ensuring device readiness. Inclusion of these systems not only improve the efficacy and accessibility of AEDs but also expands their applications in several public settings such as schools, public spaces, and workplaces.
For instance, in January 2023, Avive Solutions, Inc., received United States FDA pre-market approval (PMA) for its Avive AED, a unique Automated External Defibrillator (AED) which is designed for easy placements in variety of locations.
This emphasis of manufacturers on launch of new products aid them to diversify their products by addressing several specific needs. This factor also enables them to stay competitive, assist them to maintain their pace with the regulatory standards and meet the ever increasing expectations of the customers.
Thus, the growing emphasis of manufacturers on introduction of new, technologically advanced AEDs aid them to expand their market penetration and strengthen their presence in the market.
Growing Emphasis of Regulatory Authorities on Introduction of Public Access Bills for AEDs Surges Growing Demand for AEDs in the Market
The mandatory laws and incentivizing of placing automated external defibrillators in easy access public places such as schools, airports, sports arenas, malls, and office buildings, governments surges the demand for this life saving devices in the market. The most critical aspect of the majority of the public access bills targets improving deliverability of AEDs during emergency cases and proper training on effective use for wide diffusion.
For instance, in March 2023, in the United States, a bill was introduced to authorize the Secretary of Health and Human Services to award grants to eligible entities for the development and implementation of a comprehensive program for improving the accessibility of the AEDs in the public elementary schools and secondary schools.
This legislation promotes public safety and creates a bigger market for AED manufacturers due to the compulsion to fit these devices in schools, colleges, institutions and businesses. This regulations further expands the growth of the market as more and more regions enact similar laws and standards that call for more AED devices.
Moreover, the growing public understanding of sudden cardiac arrest and early defibrillation as lifesavers is also pushing governments to stimulate the easy availability of AEDs. This kind of policy enactment encourages innovation and competition, hence pushing the price down and bringing more affordable technology to end-users.
Development of AEDs that can deliver Multiple Aspects of Resuscitation can bring Business Opportunities to the Manufacturer
Traditional automated external defibrillators primarily focuses on defibrillation, inclusion of additional features such as a system that offers CPR feedback, real-time patient monitoring, oxygen delivery, or even advanced analytics for post-event care can make a AED a more holistic and effective resuscitation tool.
Inclusion of this expanded functionalities and capabilities in an AED increases the chances of survival and improve the quality of care which is an utmost priority for every healthcare providers and emergency responder.
This expanded capabilities attracts broader range of healthcare providers and new customer segment such as public health organizations, schools, and corporations looking to meet regulatory requirements or improve their emergency preparedness.
Manufacturers can also market this multiple aspects AEDs as a more advanced and all in one solution that reduces the need of multiple devices and reduces operational cost of an organization.
Moreover, inclusion of these additional features also bring new opportunities to manufacturers of collaborations with healthcare systems, emergency medical services, and even tech companies that can aid them to incorporate data-driven solutions like cloud-based patient monitoring, predictive analytics, and remote device management.
Therefore, the constantly evolving healthcare system towards more patient-centric and connected care models offers a unique opportunities for the AEDs that have expanded resuscitation capabilities.
Increase in Number of Product Recall Hinders the Growth of the Market
Recall of a medical device often occurs when a manufacturer identifies a defect or safety risk in the product. These recalls often create operational challenges for manufacturers, who needs to divert their resources for addressing this issues. This can slow down production and innovation as companies focus on resolving recall-related problems rather than on developing new products or enhancing existing models.
Frequent recalls often lead to negative publicity, causing potential buyers both individuals and organizations to hesitate before investing in AEDs. For instance, in May 2025, Zoll Corporation received recall of their ZOLL Powerheart G5 AED, Semi-Automatic, G5Sxxx Family Automated external defibrillator (AED) which significantly hampered their brand image in the market.
This might make healthcare providers, public facilities, and individuals very cautious while investing or replacing AED units, hence slowing market growth.
Additionally, the costs associated with managing a recall including the logistics of retrieving faulty products, repairing or replacing them, and compensating affected parties-can be financially burdensome for manufacturers, diverting resources away from research, development, and marketing of new models.
Thus, the aggregated effect of frequent recalls often lead to more inclement regulatory environment; greater stringency in the granting of product approvals, with more difficult and complex requirements for manufacturing, which further slow downs the growth of the overall market.
Companies in the Tier 1 sector account for 65.8% of the global market, ranking them as the dominant players in the industry. Tier 1 players offer a wide range of products compared to other emerging players. These companies have an established industry presence, owing to their continuous innovation.
These factors have significantly aided them to create a substantial influence in the field. Their strong financial resources enables them to enhance their research and development efforts and expand into new markets. Prominent companies within Tier 1 include Zoll Medical Corporation, Koninklijke Philips N.V., Stryker, and NIHON KOHDEN CORPORATION.
Tier 2 players dominate the industry with a 23.2% market share. Tier 2 firms have a strong focus on a specific technologies and a substantial presence in the industry, but they have less influence than Tier 1 firms.
The players are more competitive when it comes to pricing and target niche markets. They constantly focus on introducing new products and services to the growing markets. Tier 2 companies include Shenzhen Mindray Bio-Medical Electronics Co., Ltd., FUKUDA DENSHI and Schiller ag.
Tier 3 companies many of the times are limited to the local markets due to their limited production capabilities and geographical presence. Those in Tier 3 focus primarily on serving a smaller number of clients. Few of the prominent players Metrax GmbH, Avive Solutions, Inc., MEDIANA Co., Ltd.
An analysis of the automated external defibrillator industry is provided in various countries. Several regions throughout the world are examined, including Asia Pacific, North America, and Europe. United States is forecast to remain the leader with a market share of 85.7% in 2023. The market in China is anticipated to grow at a CAGR of 9.4% through 2034.
| Countries | Value CAGR (2025 to 2035) |
|---|---|
| United States | 2.5% |
| Canada | 2.8% |
| United Kingdom | 6.0% |
| Germany | 3.7% |
| India | 8.3% |
| China | 8.9% |
| South Korea | 7.9% |
Most of the states in the United States have passed legislation to include AEDs at high-risk areas, such as schools, sports arenas, gyms, and government institutions, thereby helping to increase the survival rate of sudden cardiac arrest (SCA) victims.
These laws create immense demand for AEDs since businesses and institutions are supposed to comply with the requirements because of possible legal liability and assurance of public safety.
This legislative framework encourages the installation of AEDs in public places and increases awareness of their lifesaving potential, thus encouraging more people to use them.
Therefore, the stringent laws enacted by various states in the United States to install AEDs in public places is one of the major factors that is contributing to the growth of the AED market.
These stringent mandates provides incentives and tax benefits to the installer and also aid them to maintain them. Therefore, the stringent regulatory compliance imposed by government of the United States along the growing awareness for sudden cardiac arrest further propels the growth of the AED market in the country.
Older adults are at risk of heart conditions that contribute to the incidences of SCA events; therefore, their access to lifesaving devices like automated external defibrillators increases. The more the number of aged people, the more the cases of cardiovascular diseases and conditions which include sudden cardiac arrest.
With this demographic change, the raising of installation of AEDs at locations with elderly people such as nursing homes, retirement communities, and health centers have become a sturdy call by the health care systems, public facilities, and private institutions. This has also been contributed by the increase in awareness of cardiac health and early defibrillation, which is relatively effectual in bringing up the percentage of survival.
More importantly, the German government and health organizations are laying more emphasis on installing AEDs in public places to save precious time when responding to an emergency, especially in places where the elderly population is higher. The growing health need for aged people in Germany, thus demands an increasing number of AEDs and boosts up the wheel of market growth.
Increased access to automated external defibrillators has been one of the prime drivers of growth in the AED market from the Chinese government. Aged demographics and population in the country tend to raise an increasing incidence of SCA, which stresses the need for increased access to lifesaving AED devices.
The government, for example, installs AEDs in most airports, key railway stations, sports arenas, and major shopping malls that have further access to open space. This is done out of best practice to reduce the occurrence of medical emergencies, shorten emergency response times, and improve survival rates related to cardiac events.
For instance, according to an article published by XINHUANET in January 2021, according to an action plan published in 2019, China has set goal to disseminate AEDs in all schools, government offices, public institutes, airports, shopping malls and cinemas.
Apart from that, the government has supported public education programs regarding the benefits of early defibrillation and has urged companies and institutions to consider installing AEDs as part of health and safety programs.
The fiscal obstacle to acquiring an AED for both public and private establishments was also removed by offering subsidies and other tax advantages upon buying AEDs. These initiatives taken by government in China is anticipating the growth of the AED market in the country.
| Report Attributes | Details |
|---|---|
| Market Size (2025) | USD 1.39 billion |
| Projected Market Size (2035) | USD 2.20 billion |
| CAGR (2025 to 2035) | 4.7% |
| Base Year for Estimation | 2025 |
| Historical Period | 2020 to 2025 |
| Projections Period | 2025 to 2035 |
| Quantitative Units | USD million for value and thousand units for volume |
| Product Types Analyzed (Segment 1) | Semi-Automated External Defibrillator, Automated External Defibrillator |
| Modality Types Analyzed (Segment 2) | Professional Use Automated External Defibrillators, Public Access Automated External Defibrillators |
| End-User Segments Analyzed (Segment 3) | Healthcare Settings (Hospitals, Clinics, Long Term Care Centers, Ambulatory Surgical Centers, Emergency Medical Services (EMS), Independent Catheterization Labs, Urgent Care Centers), Public Access (Airports, Train Stations, Shopping Malls, Hypermarket & Supermarket, Sports Arenas/Stadiums, Schools & Colleges, Gymnasiums, Corporate Office Buildings, Home Care Settings, Parks, Hotels & Restaurants, Others) |
| Regions Covered | North America, Latin America, Western Europe, Eastern Europe, South Asia & Pacific, East Asia, Middle East & Africa |
| Countries Covered | United States, Canada, Brazil, Mexico, Germany, France, United Kingdom, Italy, Spain, China, India, Japan, South Korea, Australia, GCC Countries, South Africa |
| Key Players influencing the Automated External Defibrillator Market | Zoll Medical Corporation, Koninklijke Philips N.V., Stryker, NIHON KOHDEN CORPORATION, Shenzhen Mindray Bio-Medical Electronics Co., Ltd., FUKUDA DENSHI, Schiller AG, Metrax GmbH, Avive Solutions, Inc., MEDIANA CO., Ltd., Others |
| Additional Attributes | Sales by product and modality type, Growth in public access defibrillator installations, Regional regulations and safety standards, Trends in automated defibrillator technology, Role of defibrillators in emergency medical services, Impact of public health campaigns |
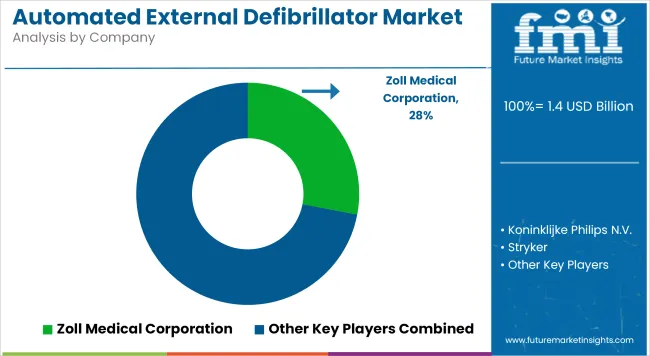
In terms of product, the industry is segregated into semi-automated external defibrillator and automated external defibrillator
In terms of modality, the industry is segregated into professional use automated external defibrillators and public access automated external defibrillators
In terms of end user, the industry is segmented into healthcare settings (hospitals, clinics, long term care centers, ambulatory surgical centers, emergency medical services (EMS), independent catheterization labs and urgent care centers) and public access (airports, train stations, shopping malls, hypermarket & supermarket, sports arenas/stadiums, schools & colleges, gymnasiums, corporate office buildings, home care settings, parks, hotels & restaurants and others)
Key countries of North America, Latin America, Western Europe, Eastern Europe, South Asia and Pacific, East Asia, Middle East, and Africa have been covered in the report.
Automated External Defibrillator are expected to increase at a CAGR of 4.7% between 2025 and 2035.
The fully automated external defibrillator segment is expected to occupy 85.4% market share in 2025.
The market for automated external defibrillator is expected to reach USD 2,097.1 million by 2035.
The United States is forecast to see a CAGR of 2.5% during the assessment period.
The key players in the Automated External Defibrillator industry include Zoll Medical Corporation, Koninklijke Philips N.V., Stryker, NIHON KOHDEN CORPORATION, Shenzhen Mindray Bio-Medical Electronics Co., Ltd., FUKUDA DENSHI, Schiller ag, Metrax GmbH, Avive Solutions, Inc., MEDIANA Co., Ltd., Progetti Medical Equipment Solutions, Bexen, Corpuls, CU Medical Systems, Inc., Promed Technology Co., Limited, INNOMED MEDICAL INC.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Automated Machine Learning Market Forecast Outlook 2025 to 2035
Automated CPR Device Market Size and Share Forecast Outlook 2025 to 2035
Automated Test Equipment Market Size and Share Forecast Outlook 2025 to 2035
Automated Compound Storage and Retrieval (ACSR) Market Size and Share Forecast Outlook 2025 to 2035
Automated People Mover Market Size and Share Forecast Outlook 2025 to 2035
Automated Colony Picking Systems Market Size and Share Forecast Outlook 2025 to 2035
Automated Truck Loading System Market Size and Share Forecast Outlook 2025 to 2035
Automated Microplate Handling Systems Market Size and Share Forecast Outlook 2025 to 2035
Automated Solid Phase Extraction Systems Market Size and Share Forecast Outlook 2025 to 2035
Automated Infrastructure Management Solution Market Size and Share Forecast Outlook 2025 to 2035
Automated Mineralogy Solution Market Size and Share Forecast Outlook 2025 to 2035
Automated Material Handling Equipment Market Size and Share Forecast Outlook 2025 to 2035
Automated Feeding Systems Market Size and Share Forecast Outlook 2025 to 2035
Automated Labeling Machines Market Size and Share Forecast Outlook 2025 to 2035
Automated Solar Panel Cleaning Market Size and Share Forecast Outlook 2025 to 2035
Automated Molecular Diagnostics Testing System Market Size and Share Forecast Outlook 2025 to 2035
Automated Infrastructure Management (AIM) Solutions Market Size and Share Forecast Outlook 2025 to 2035
Automated Window Blinds Market Size and Share Forecast Outlook 2025 to 2035
Automated Cell Culture Systems Market Analysis - Size, Share & Forecast 2025-2035
Automated Cell Biology Systems Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA