The Atypical Hemolytic Uremic Syndrome (aHUS) Treatment market is valued at USD 1.43 billion in 2025. As per FMI’s analysis, the aHUS Treatment market will grow at a CAGR of 4.9% and will reach USD 2.31 billion by 2035. The treatment landscape of aHUS worldwide has experienced steady advancements, with an emphasis on creating targeted therapies, such as complement inhibitors, immunotherapies, plasma exchange, and supportive care.
In 2024, the Atypical Hemolytic Uremic Syndrome (aHUS) Treatment industry maintained its growth pattern driven by advances in medical science and increased awareness of the condition. The industry experienced rising adoption of complement inhibitors such as eculizumab and ravulizumab, which are still the main treatment methods. Plasma exchange, plasma infusion, and immunotherapy also helped to enhance patient outcomes.
In 2025, the aHUS treatment industry will continue to grow. With advancements in more sensitive diagnostic tests, increasing numbers of aHUS cases will be identified, propelling increased treatment demand. New, targeted therapies, most notably complement inhibitors, will further redefine the treatment environment. Government support for research into rare diseases, alongside pharmaceutical advancements, will promote segment growth.
| Metric | Value |
|---|---|
| Industry Value (2025E) | USD 1.43 billion |
| Industry Value (2035F) | USD 2.31 billion |
| CAGR (2025 to 2035) | 4.9% |
The Atypical Hemolytic Uremic Syndrome (aHUS) Treatment segment is poised for steady growth, driven by advances in diagnostic technology and precision therapies such as complement inhibitors. Pharmaceutical firms and healthcare providers that specialize in rare diseases are likely to benefit substantially from these advances. However, underdeveloped regions with less healthcare infrastructure might experience difficulties in accessing these new treatments, and hence, might restrain their growth in the sector.
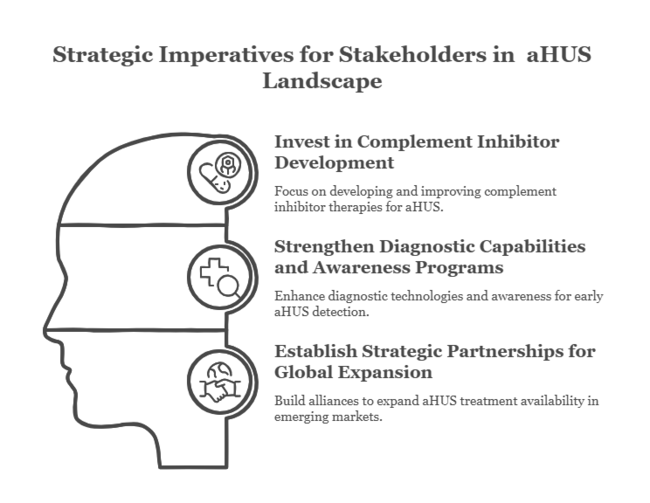
Invest in Complement Inhibitor Development
Investors must focus on investing in the development and growth of complement inhibitor therapies, as they are emerging as the foundation of aHUS treatment. Emphasize the improvement of the efficacy and availability of these therapies to meet increasing patient demand.
Strengthen Diagnostic Capabilities and Awareness Programs
With the growing incidence of aHUS, in line with the development of diagnostic technologies and growing awareness among healthcare professionals and patients, it is imperative to become important. This will promote early diagnosis and timely treatment, fostering sector penetration.
Establish Strategic Partnerships for Global Expansion
Stakeholders must look into strategic alliances, especially in emerging sectors such as Asia Pacific, to boost distribution channels and improve the availability of aHUS treatments. Teamwork with local research institutions and healthcare systems can be useful in gaining insights and enhancing treatment availability.
| Risk | Probability-Impact |
|---|---|
| Regulatory Barriers - Regulatory delays in approvals may delay the introduction of new therapies, constraining industry expansion. | High-High |
| Restricted Access in Developing Sectors - Inadequate healthcare infrastructure in some areas could limit the availability of treatment and industry growth. | Medium-High |
| Emerging Therapies Competition - More effective, newer therapies can drive industry share eroding competition. | Medium-Medium |
| Priority | Immediate Action |
|---|---|
| Regulatory Approvals | Streamline regulatory procedures to accelerate new aHUS treatment approvals. |
| Emerging Industry Penetration | Form strategic alliances in Asia Pacific to build distribution and access. |
| Competitive Intelligence | Regularly observe innovations by competitors and make strategies accordingly. |
To stay ahead, companies need to focus on speeding up regulatory approval processes and investing in the emerging sectors of Asia Pacific to unlock growth potential. Tightening local partnerships with healthcare providers and strengthening distribution networks will be key to driving industry penetration.
At the same time, closely monitoring the emerging competition and changing treatment technologies will allow for quick strategic tweaks to maintain industry leadership in the fast-changing aHUS treatment sector. By coordinating their roadmap with these strategic imperatives, companies can gain a competitive advantage in an expanding sector and leverage the rising demand for innovative aHUS treatments.
The aHUS treatment industry product type segment is anticipated to register strong growth during the forecast period. Mono products, meant for single-therapy usage, are anticipated to enjoy a moderate CAGR while demand for dedicated, specialized treatments grows. Combination products, which provide a more holistic treatment, are anticipated to record a higher growth rate because they are capable of tackling several areas of aHUS pathology.
The convergence of mono and combination therapies is picking up pace as a hybrid therapy targeting both targeted and broad-based requirements. The overall product type category is anticipated to rise at a CAGR of 5.3% fueled by developments in drug delivery forms and customized treatments.
The type of molecule segment, which includes monoclonal antibodies, peptides, polymers, small molecules, and gene therapies, is poised to experience tremendous growth. Monoclonal antibodies will be the leaders in this space with an excellent pace as drugs such as eculizumab remain the standard for the treatment of aHUS. Peptides and small molecules are also expected to increase at a moderate pace as novel drugs get launched with mechanism-specific action.
Gene therapies are showing up as a novel solution, with a high CAGR due to their capability to offer long-term treatment options for aHUS patients. The total molecule type segment will grow at a CAGR of 5.0% through innovation in therapeutic technologies.
The route of administration segment is anticipated to increase steadily over the forecast period. Parenteral administration, such as intravenous and subcutaneous routes, will continue to be the leading route because of the immediate and controlled release of aHUS treatments. Intravenous administration, specifically, is anticipated to experience considerable growth because of its direct effect on quickly treating the condition.
Oral treatments, while presently less prevalent, will experience incremental growth as new therapies emerge. Topical treatments, although still specialty, might experience moderate growth with improved drug delivery systems. The total route of administration segment will expand at a CAGR of 4.8%, with parenteral techniques leading the charge.
The channel segment of the distribution will continue to develop as access to healthcare increases worldwide. Hospital pharmacies will continue to be the main channel of distribution because of their involvement in providing specialty treatment for aHUS patients. Retail pharmacies will experience constant growth as more awareness is generated and treatments become available.
Online pharmacies, spurred by healthcare's digital revolution, will have the fastest growth rate, giving patients greater ease of access to aHUS treatments. This segment is anticipated to expand at a CAGR of 5.1%, with online pharmacies experiencing the most significant increase as a result of the growing trend towards telehealth and home-based care solutions.
The United States is a leading country in the treatment industry of aHUS, led by increased healthcare expenditure, sophisticated medical research, and strong healthcare infrastructure. An established presence of major pharmaceutical companies and active clinical trials for new drugs increases the uptake of novel drugs, specifically complement inhibitors. Great numbers of patients get diagnosed because of extensive awareness and diagnostic facilities.
Nevertheless, the sector is challenged by high treatment costs and rural access problems for healthcare. Growth will be fueled by growing emphasis on rare disease therapies and government efforts to enhance patient access. FMI opines that the CAGR of the USA is projected to be 5.2% during the forecast period.
India's sector for aHUS treatment is expected to experience steady growth as a result of enhancing healthcare infrastructure, increasing awareness, and increasing the prevalence of rare diseases. Limited access to specialized care and high prices are challenges that exist in the country, but more government efforts toward treating rare diseases are expected to counteract these hurdles.
Increasing pharmaceutical activity and a growing middle class will further drive industry growth. New therapies, such as complement inhibitors, are anticipated to be used increasingly as accessibility to healthcare becomes better.
FMI forecasts that the aHUS treatment industry in India is projected to grow at a CAGR of 5.0% between 2025 and 2035.
China, with its immense population and developing healthcare system, offers a massive opportunity for the aHUS treatment industry. Growing awareness and development in diagnostic technologies are anticipated to lead to early diagnosis, which will also drive treatment demand.
The efforts of the government to enhance healthcare and offer increased access to therapies for rare diseases will further push the sector toward growth. Obstacles still lie in the aspects of affordability as well as accessibility of specialized medical professionals.
FMI projects that the market for aHUS treatments in China is expected to grow at a CAGR of 5.1% from 2025 to 2035.
The United Kingdom industry for the treatment of aHUS will further grow as a result of its established healthcare system and increasing focus on treating rare diseases. National Health Service (NHS) has been keen in funding rare disease treatments, which is to the advantage of patients with aHUS.
In addition, there is a high degree of healthcare awareness and diagnostic skill enabling the detection and treatment of the disease at an early stage. Nevertheless, regulatory hurdles post-Brexit could influence access to new therapies.
FMI opines that the UK industry is expected to grow at a CAGR of 4.9% from 2025 to 2035.
Germany's aHUS treatment industry is set to grow steadily because of its well-developed healthcare infrastructure and focus on the management of rare diseases. The nation's advanced regulatory system facilitates approval for innovative therapies, and a developed pharmaceutical sector ensures access to novel drugs at the right time.
Nevertheless, high treatment expenses and healthcare inequalities among regions may be an issue. Increasing awareness and improved diagnostic standards are likely to result in earlier diagnoses, fueling treatment demand.
FMI projects that the aHUS treatment industry in Germany is expected to grow at a CAGR of 4.8% from 2025 to 2035.
South Korea's aHUS therapy industry is expanding steadily, fueled by its highly developed healthcare system and mounting awareness of rare diseases. South Korea's high focus on research and development, especially in biotechnology, fuels the creation of innovative treatments for aHUS.
As South Korea expands access to healthcare services, even though there are issues with patient access to costly therapies, it is likely to experience continued expansion. The government's efforts to advance rare disease treatments and enhance diagnostic accuracy will also propel market growth.
FMI opines that the CAGR of South Korea's aHUS treatment industry is projected to grow at a CAGR of 5.0% between 2025 and 2035.
Japan's aHUS treatment industry is poised for sustained growth in light of its sophisticated healthcare infrastructure and superior patient awareness. Japan is at the forefront of medical technology, with regular developments in diagnostic techniques and therapy.
Encouragement from the government towards rare disease research and the approval of novel therapies for aHUS are likely to drive industry growth. Yet, Japan has difficulties in tackling the expense of therapies and guaranteeing equal access to treatments in rural regions.
FMI forecasts that the industry for aHUS treatments in Japan is projected to grow at a CAGR of 5.1% from 2025 to 2035.
France's aHUS treatment industry would grow steadily because of its advanced healthcare system and increasing patient population. The emphasis of the French healthcare system to give equal access to medical treatments, including therapies for rare diseases, would promote sector growth. In addition, higher awareness and early detection will result in increased adoption of treatments. Nonetheless, the nation will be challenged by the high cost of treatments, especially in the scope of healthcare expenditure budgets.
FMI opines that France aHUS industry will grow at a CAGR of 4.7%. during the forecast period.
Italy's aHUS treatment industry is likely to grow steadily, aided by advancements in the diagnosis of rare diseases and an established healthcare infrastructure. The nation's healthcare setup facilitates relatively easy access to sophisticated treatments, while regional variations in access to care are still an issue.
Growing awareness of aHUS coupled with the presence of complement inhibitors are likely to spur segment growth. Support from government initiatives for rare diseases and more comprehensive treatment policies will also facilitate growth of this segment.
FMI forecasts that the aHUS treatment sector in Italy is expected to grow at a CAGR of 4.8% from 2025 to 2035.
Australia and New Zealand provide a suitable setting for the aHUS treatment sector because of their well-established healthcare systems and rising emphasis on rare diseases. Both nations possess advanced diagnostic facilities and are witnessing increased uptake of new therapies. The encouragement of rare disease research by the government and financing for treatments will drive the growth of the industry.
Nevertheless, the exorbitant price of therapies could compromise accessibility for a few patients. With improving healthcare access, demand for the treatment of aHUS will keep increasing.
FMI opines that the CAGR of the Australia-New Zealand aHUS industry will reach 5.0% during the forecast period.
| Countries | Government Regulations |
|---|---|
| USA | FDA approval is mandatory for aHUS treatments, with Orphan Drug Designation providing incentives. Companies have to comply with strict clinical trial and data requirements. |
| India | The CDSCO oversees approvals under the Drugs and Cosmetics Act. Treatments have to be tested for safety and efficacy. |
| China | The NMPA oversees drug approvals, and foreign companies require local partners for distribution. |
| UK | The MHRA oversees aHUS treatment approvals, with compliance with EU regulations until post-Brexit adjustments. |
| Germany | The BfArM regulates approvals of aHUS treatments. Biologics and orphan drug treatments have strict regulations. |
| South Korea | The MFDS regulates treatments and data submission for clinical trials and safety assessments. |
| Japan | The PMDA regulates approvals with particular interest in clinical trials and local production requirements for quicker access. |
| France | ANSM regulates treatments of aHUS, with stringent regulation of safety and clinical trials. Reimbursement of treatments is determined by regulatory evaluation. |
| Italy | AIFA controls approvals, specifically for orphan drugs and post-clinical trials sector access. |
| Australia-New Zealand | TGA controls treatments, and there are specific requirements to meet for biologics and orphan drugs in terms of approval, such as local clinical trials. |
The industry for Atypical Hemolytic Uremic Syndrome (aHUS) treatment is moderately concentrated with major players such as Novartis, Alexion Pharmaceuticals, and Roche competing actively in the sector through competitive pricing, innovation, collaborations, and international expansion.
Leading firms are using a mix of competitive pricing, ongoing innovation, strategic alliances, and international expansion to consolidate their positions in the aHUS treatment sector. For example, Novartis has increased its midterm sales outlook, expecting higher revenue from priority drugs and new product launches as it seeks to become one of the USA's top five pharmaceutical firms.
In June 2024, Novartis finished acquiring MorphoSys for EUR 2.7 billion and acquired a pipeline of antibody-drug candidates, including potential treatments for aHUS.
Likewise, in January 2024, Roche bought Carmot Therapeutics for USD 3.1 billion, bringing in three clinical-stage compounds for treating obesity and diabetes.
Alexion Pharmaceuticals (AstraZeneca) - ~55-60%
Leads with Soliris (eculizumab) and Ultomiris (ravulizumab), the standard-of-care complement inhibitors.
Novartis AG - ~20-25%
Sectors LNP023 (iptacopan), a lead oral Factor B inhibitor in late-stage trials for aHUS.
Roche - ~8-12%
Develops RG6107 (anti-factor D antibody), a potential alternative to Soliris/Ultomiris.
Apellis Pharmaceuticals - ~5-8%
Investigational pegcetacoplan (versus C3) for complement-mediated diseases, including aHUS.
BioCryst Pharmaceuticals - ~3-5%
Developing BCX9930 (oral Factor D inhibitor) as next-gen aHUS therapy.
ChemoCentryx (Vifor Pharma) - ~2-4%
Investigating avacopan (complement C5a receptor inhibitor) for aHUS.
Mono, Combination, Mono/Combination
Monoclonal Antibody, Peptides, Polymer, Small molecule, Gene therapy,
Oral, Parenteral, Intravenous, Subcutaneous, Topical
Hospital Pharmacy, Retail Pharmacy, Online Pharmacy
North America, Latin America, Europe, South Asia, East Asia, Oceania, MEA
Improvements in complement inhibitors and increased awareness of aHUS are enhancing diagnosis and increasing treatment opportunities.
Innovators are concentrating on creating targeted therapies and establishing strategic collaborations to advance drug development and increase patient access.
Approval regulations for medications such as eculizumab are essential for improving treatment coverage and meeting aHUS patient urgent needs.
Healthcare professionals are incorporating more recent therapies, including eculizumab and ravulizumab, into care protocols, producing improved patient results.
Increased investment is spurring the advancement of better therapies, and at the same time, deeperening the comprehension of aHUS's fundamental mechanisms.
Table 1: Global Market Value (US$ Million) Forecast by Region, 2018 to 2033
Table 2: Global Market Value (US$ Million) Forecast by Product Type, 2018 to 2033
Table 3: Global Market Value (US$ Million) Forecast by Molecule Type, 2018 to 2033
Table 4: Global Market Value (US$ Million) Forecast by Route of Administration , 2018 to 2033
Table 5: Global Market Value (US$ Million) Forecast by Distribution Channel, 2018 to 2033
Table 6: North America Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 7: North America Market Value (US$ Million) Forecast by Product Type, 2018 to 2033
Table 8: North America Market Value (US$ Million) Forecast by Molecule Type, 2018 to 2033
Table 9: North America Market Value (US$ Million) Forecast by Route of Administration , 2018 to 2033
Table 10: North America Market Value (US$ Million) Forecast by Distribution Channel, 2018 to 2033
Table 11: Latin America Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 12: Latin America Market Value (US$ Million) Forecast by Product Type, 2018 to 2033
Table 13: Latin America Market Value (US$ Million) Forecast by Molecule Type, 2018 to 2033
Table 14: Latin America Market Value (US$ Million) Forecast by Route of Administration , 2018 to 2033
Table 15: Latin America Market Value (US$ Million) Forecast by Distribution Channel, 2018 to 2033
Table 16: Europe Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 17: Europe Market Value (US$ Million) Forecast by Product Type, 2018 to 2033
Table 18: Europe Market Value (US$ Million) Forecast by Molecule Type, 2018 to 2033
Table 19: Europe Market Value (US$ Million) Forecast by Route of Administration , 2018 to 2033
Table 20: Europe Market Value (US$ Million) Forecast by Distribution Channel, 2018 to 2033
Table 21: South Asia Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 22: South Asia Market Value (US$ Million) Forecast by Product Type, 2018 to 2033
Table 23: South Asia Market Value (US$ Million) Forecast by Molecule Type, 2018 to 2033
Table 24: South Asia Market Value (US$ Million) Forecast by Route of Administration , 2018 to 2033
Table 25: South Asia Market Value (US$ Million) Forecast by Distribution Channel, 2018 to 2033
Table 26: East Asia Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 27: East Asia Market Value (US$ Million) Forecast by Product Type, 2018 to 2033
Table 28: East Asia Market Value (US$ Million) Forecast by Molecule Type, 2018 to 2033
Table 29: East Asia Market Value (US$ Million) Forecast by Route of Administration , 2018 to 2033
Table 30: East Asia Market Value (US$ Million) Forecast by Distribution Channel, 2018 to 2033
Table 31: Oceania Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 32: Oceania Market Value (US$ Million) Forecast by Product Type, 2018 to 2033
Table 33: Oceania Market Value (US$ Million) Forecast by Molecule Type, 2018 to 2033
Table 34: Oceania Market Value (US$ Million) Forecast by Route of Administration , 2018 to 2033
Table 35: Oceania Market Value (US$ Million) Forecast by Distribution Channel, 2018 to 2033
Table 36: MEA Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 37: MEA Market Value (US$ Million) Forecast by Product Type, 2018 to 2033
Table 38: MEA Market Value (US$ Million) Forecast by Molecule Type, 2018 to 2033
Table 39: MEA Market Value (US$ Million) Forecast by Route of Administration , 2018 to 2033
Table 40: MEA Market Value (US$ Million) Forecast by Distribution Channel, 2018 to 2033
Figure 1: Global Market Value (US$ Million) by Product Type, 2023 to 2033
Figure 2: Global Market Value (US$ Million) by Molecule Type, 2023 to 2033
Figure 3: Global Market Value (US$ Million) by Route of Administration , 2023 to 2033
Figure 4: Global Market Value (US$ Million) by Distribution Channel, 2023 to 2033
Figure 5: Global Market Value (US$ Million) by Region, 2023 to 2033
Figure 6: Global Market Value (US$ Million) Analysis by Region, 2018 to 2033
Figure 7: Global Market Value Share (%) and BPS Analysis by Region, 2023 to 2033
Figure 8: Global Market Y-o-Y Growth (%) Projections by Region, 2023 to 2033
Figure 9: Global Market Value (US$ Million) Analysis by Product Type, 2018 to 2033
Figure 10: Global Market Value Share (%) and BPS Analysis by Product Type, 2023 to 2033
Figure 11: Global Market Y-o-Y Growth (%) Projections by Product Type, 2023 to 2033
Figure 12: Global Market Value (US$ Million) Analysis by Molecule Type, 2018 to 2033
Figure 13: Global Market Value Share (%) and BPS Analysis by Molecule Type, 2023 to 2033
Figure 14: Global Market Y-o-Y Growth (%) Projections by Molecule Type, 2023 to 2033
Figure 15: Global Market Value (US$ Million) Analysis by Route of Administration , 2018 to 2033
Figure 16: Global Market Value Share (%) and BPS Analysis by Route of Administration , 2023 to 2033
Figure 17: Global Market Y-o-Y Growth (%) Projections by Route of Administration , 2023 to 2033
Figure 18: Global Market Value (US$ Million) Analysis by Distribution Channel, 2018 to 2033
Figure 19: Global Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 20: Global Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 21: Global Market Attractiveness by Product Type, 2023 to 2033
Figure 22: Global Market Attractiveness by Molecule Type, 2023 to 2033
Figure 23: Global Market Attractiveness by Route of Administration , 2023 to 2033
Figure 24: Global Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 25: Global Market Attractiveness by Region, 2023 to 2033
Figure 26: North America Market Value (US$ Million) by Product Type, 2023 to 2033
Figure 27: North America Market Value (US$ Million) by Molecule Type, 2023 to 2033
Figure 28: North America Market Value (US$ Million) by Route of Administration , 2023 to 2033
Figure 29: North America Market Value (US$ Million) by Distribution Channel, 2023 to 2033
Figure 30: North America Market Value (US$ Million) by Country, 2023 to 2033
Figure 31: North America Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 32: North America Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 33: North America Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 34: North America Market Value (US$ Million) Analysis by Product Type, 2018 to 2033
Figure 35: North America Market Value Share (%) and BPS Analysis by Product Type, 2023 to 2033
Figure 36: North America Market Y-o-Y Growth (%) Projections by Product Type, 2023 to 2033
Figure 37: North America Market Value (US$ Million) Analysis by Molecule Type, 2018 to 2033
Figure 38: North America Market Value Share (%) and BPS Analysis by Molecule Type, 2023 to 2033
Figure 39: North America Market Y-o-Y Growth (%) Projections by Molecule Type, 2023 to 2033
Figure 40: North America Market Value (US$ Million) Analysis by Route of Administration , 2018 to 2033
Figure 41: North America Market Value Share (%) and BPS Analysis by Route of Administration , 2023 to 2033
Figure 42: North America Market Y-o-Y Growth (%) Projections by Route of Administration , 2023 to 2033
Figure 43: North America Market Value (US$ Million) Analysis by Distribution Channel, 2018 to 2033
Figure 44: North America Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 45: North America Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 46: North America Market Attractiveness by Product Type, 2023 to 2033
Figure 47: North America Market Attractiveness by Molecule Type, 2023 to 2033
Figure 48: North America Market Attractiveness by Route of Administration , 2023 to 2033
Figure 49: North America Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 50: North America Market Attractiveness by Country, 2023 to 2033
Figure 51: Latin America Market Value (US$ Million) by Product Type, 2023 to 2033
Figure 52: Latin America Market Value (US$ Million) by Molecule Type, 2023 to 2033
Figure 53: Latin America Market Value (US$ Million) by Route of Administration , 2023 to 2033
Figure 54: Latin America Market Value (US$ Million) by Distribution Channel, 2023 to 2033
Figure 55: Latin America Market Value (US$ Million) by Country, 2023 to 2033
Figure 56: Latin America Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 57: Latin America Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 58: Latin America Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 59: Latin America Market Value (US$ Million) Analysis by Product Type, 2018 to 2033
Figure 60: Latin America Market Value Share (%) and BPS Analysis by Product Type, 2023 to 2033
Figure 61: Latin America Market Y-o-Y Growth (%) Projections by Product Type, 2023 to 2033
Figure 62: Latin America Market Value (US$ Million) Analysis by Molecule Type, 2018 to 2033
Figure 63: Latin America Market Value Share (%) and BPS Analysis by Molecule Type, 2023 to 2033
Figure 64: Latin America Market Y-o-Y Growth (%) Projections by Molecule Type, 2023 to 2033
Figure 65: Latin America Market Value (US$ Million) Analysis by Route of Administration , 2018 to 2033
Figure 66: Latin America Market Value Share (%) and BPS Analysis by Route of Administration , 2023 to 2033
Figure 67: Latin America Market Y-o-Y Growth (%) Projections by Route of Administration , 2023 to 2033
Figure 68: Latin America Market Value (US$ Million) Analysis by Distribution Channel, 2018 to 2033
Figure 69: Latin America Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 70: Latin America Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 71: Latin America Market Attractiveness by Product Type, 2023 to 2033
Figure 72: Latin America Market Attractiveness by Molecule Type, 2023 to 2033
Figure 73: Latin America Market Attractiveness by Route of Administration , 2023 to 2033
Figure 74: Latin America Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 75: Latin America Market Attractiveness by Country, 2023 to 2033
Figure 76: Europe Market Value (US$ Million) by Product Type, 2023 to 2033
Figure 77: Europe Market Value (US$ Million) by Molecule Type, 2023 to 2033
Figure 78: Europe Market Value (US$ Million) by Route of Administration , 2023 to 2033
Figure 79: Europe Market Value (US$ Million) by Distribution Channel, 2023 to 2033
Figure 80: Europe Market Value (US$ Million) by Country, 2023 to 2033
Figure 81: Europe Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 82: Europe Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 83: Europe Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 84: Europe Market Value (US$ Million) Analysis by Product Type, 2018 to 2033
Figure 85: Europe Market Value Share (%) and BPS Analysis by Product Type, 2023 to 2033
Figure 86: Europe Market Y-o-Y Growth (%) Projections by Product Type, 2023 to 2033
Figure 87: Europe Market Value (US$ Million) Analysis by Molecule Type, 2018 to 2033
Figure 88: Europe Market Value Share (%) and BPS Analysis by Molecule Type, 2023 to 2033
Figure 89: Europe Market Y-o-Y Growth (%) Projections by Molecule Type, 2023 to 2033
Figure 90: Europe Market Value (US$ Million) Analysis by Route of Administration , 2018 to 2033
Figure 91: Europe Market Value Share (%) and BPS Analysis by Route of Administration , 2023 to 2033
Figure 92: Europe Market Y-o-Y Growth (%) Projections by Route of Administration , 2023 to 2033
Figure 93: Europe Market Value (US$ Million) Analysis by Distribution Channel, 2018 to 2033
Figure 94: Europe Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 95: Europe Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 96: Europe Market Attractiveness by Product Type, 2023 to 2033
Figure 97: Europe Market Attractiveness by Molecule Type, 2023 to 2033
Figure 98: Europe Market Attractiveness by Route of Administration , 2023 to 2033
Figure 99: Europe Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 100: Europe Market Attractiveness by Country, 2023 to 2033
Figure 101: South Asia Market Value (US$ Million) by Product Type, 2023 to 2033
Figure 102: South Asia Market Value (US$ Million) by Molecule Type, 2023 to 2033
Figure 103: South Asia Market Value (US$ Million) by Route of Administration , 2023 to 2033
Figure 104: South Asia Market Value (US$ Million) by Distribution Channel, 2023 to 2033
Figure 105: South Asia Market Value (US$ Million) by Country, 2023 to 2033
Figure 106: South Asia Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 107: South Asia Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 108: South Asia Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 109: South Asia Market Value (US$ Million) Analysis by Product Type, 2018 to 2033
Figure 110: South Asia Market Value Share (%) and BPS Analysis by Product Type, 2023 to 2033
Figure 111: South Asia Market Y-o-Y Growth (%) Projections by Product Type, 2023 to 2033
Figure 112: South Asia Market Value (US$ Million) Analysis by Molecule Type, 2018 to 2033
Figure 113: South Asia Market Value Share (%) and BPS Analysis by Molecule Type, 2023 to 2033
Figure 114: South Asia Market Y-o-Y Growth (%) Projections by Molecule Type, 2023 to 2033
Figure 115: South Asia Market Value (US$ Million) Analysis by Route of Administration , 2018 to 2033
Figure 116: South Asia Market Value Share (%) and BPS Analysis by Route of Administration , 2023 to 2033
Figure 117: South Asia Market Y-o-Y Growth (%) Projections by Route of Administration , 2023 to 2033
Figure 118: South Asia Market Value (US$ Million) Analysis by Distribution Channel, 2018 to 2033
Figure 119: South Asia Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 120: South Asia Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 121: South Asia Market Attractiveness by Product Type, 2023 to 2033
Figure 122: South Asia Market Attractiveness by Molecule Type, 2023 to 2033
Figure 123: South Asia Market Attractiveness by Route of Administration , 2023 to 2033
Figure 124: South Asia Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 125: South Asia Market Attractiveness by Country, 2023 to 2033
Figure 126: East Asia Market Value (US$ Million) by Product Type, 2023 to 2033
Figure 127: East Asia Market Value (US$ Million) by Molecule Type, 2023 to 2033
Figure 128: East Asia Market Value (US$ Million) by Route of Administration , 2023 to 2033
Figure 129: East Asia Market Value (US$ Million) by Distribution Channel, 2023 to 2033
Figure 130: East Asia Market Value (US$ Million) by Country, 2023 to 2033
Figure 131: East Asia Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 132: East Asia Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 133: East Asia Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 134: East Asia Market Value (US$ Million) Analysis by Product Type, 2018 to 2033
Figure 135: East Asia Market Value Share (%) and BPS Analysis by Product Type, 2023 to 2033
Figure 136: East Asia Market Y-o-Y Growth (%) Projections by Product Type, 2023 to 2033
Figure 137: East Asia Market Value (US$ Million) Analysis by Molecule Type, 2018 to 2033
Figure 138: East Asia Market Value Share (%) and BPS Analysis by Molecule Type, 2023 to 2033
Figure 139: East Asia Market Y-o-Y Growth (%) Projections by Molecule Type, 2023 to 2033
Figure 140: East Asia Market Value (US$ Million) Analysis by Route of Administration , 2018 to 2033
Figure 141: East Asia Market Value Share (%) and BPS Analysis by Route of Administration , 2023 to 2033
Figure 142: East Asia Market Y-o-Y Growth (%) Projections by Route of Administration , 2023 to 2033
Figure 143: East Asia Market Value (US$ Million) Analysis by Distribution Channel, 2018 to 2033
Figure 144: East Asia Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 145: East Asia Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 146: East Asia Market Attractiveness by Product Type, 2023 to 2033
Figure 147: East Asia Market Attractiveness by Molecule Type, 2023 to 2033
Figure 148: East Asia Market Attractiveness by Route of Administration , 2023 to 2033
Figure 149: East Asia Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 150: East Asia Market Attractiveness by Country, 2023 to 2033
Figure 151: Oceania Market Value (US$ Million) by Product Type, 2023 to 2033
Figure 152: Oceania Market Value (US$ Million) by Molecule Type, 2023 to 2033
Figure 153: Oceania Market Value (US$ Million) by Route of Administration , 2023 to 2033
Figure 154: Oceania Market Value (US$ Million) by Distribution Channel, 2023 to 2033
Figure 155: Oceania Market Value (US$ Million) by Country, 2023 to 2033
Figure 156: Oceania Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 157: Oceania Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 158: Oceania Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 159: Oceania Market Value (US$ Million) Analysis by Product Type, 2018 to 2033
Figure 160: Oceania Market Value Share (%) and BPS Analysis by Product Type, 2023 to 2033
Figure 161: Oceania Market Y-o-Y Growth (%) Projections by Product Type, 2023 to 2033
Figure 162: Oceania Market Value (US$ Million) Analysis by Molecule Type, 2018 to 2033
Figure 163: Oceania Market Value Share (%) and BPS Analysis by Molecule Type, 2023 to 2033
Figure 164: Oceania Market Y-o-Y Growth (%) Projections by Molecule Type, 2023 to 2033
Figure 165: Oceania Market Value (US$ Million) Analysis by Route of Administration , 2018 to 2033
Figure 166: Oceania Market Value Share (%) and BPS Analysis by Route of Administration , 2023 to 2033
Figure 167: Oceania Market Y-o-Y Growth (%) Projections by Route of Administration , 2023 to 2033
Figure 168: Oceania Market Value (US$ Million) Analysis by Distribution Channel, 2018 to 2033
Figure 169: Oceania Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 170: Oceania Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 171: Oceania Market Attractiveness by Product Type, 2023 to 2033
Figure 172: Oceania Market Attractiveness by Molecule Type, 2023 to 2033
Figure 173: Oceania Market Attractiveness by Route of Administration , 2023 to 2033
Figure 174: Oceania Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 175: Oceania Market Attractiveness by Country, 2023 to 2033
Figure 176: MEA Market Value (US$ Million) by Product Type, 2023 to 2033
Figure 177: MEA Market Value (US$ Million) by Molecule Type, 2023 to 2033
Figure 178: MEA Market Value (US$ Million) by Route of Administration , 2023 to 2033
Figure 179: MEA Market Value (US$ Million) by Distribution Channel, 2023 to 2033
Figure 180: MEA Market Value (US$ Million) by Country, 2023 to 2033
Figure 181: MEA Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 182: MEA Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 183: MEA Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 184: MEA Market Value (US$ Million) Analysis by Product Type, 2018 to 2033
Figure 185: MEA Market Value Share (%) and BPS Analysis by Product Type, 2023 to 2033
Figure 186: MEA Market Y-o-Y Growth (%) Projections by Product Type, 2023 to 2033
Figure 187: MEA Market Value (US$ Million) Analysis by Molecule Type, 2018 to 2033
Figure 188: MEA Market Value Share (%) and BPS Analysis by Molecule Type, 2023 to 2033
Figure 189: MEA Market Y-o-Y Growth (%) Projections by Molecule Type, 2023 to 2033
Figure 190: MEA Market Value (US$ Million) Analysis by Route of Administration , 2018 to 2033
Figure 191: MEA Market Value Share (%) and BPS Analysis by Route of Administration , 2023 to 2033
Figure 192: MEA Market Y-o-Y Growth (%) Projections by Route of Administration , 2023 to 2033
Figure 193: MEA Market Value (US$ Million) Analysis by Distribution Channel, 2018 to 2033
Figure 194: MEA Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 195: MEA Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 196: MEA Market Attractiveness by Product Type, 2023 to 2033
Figure 197: MEA Market Attractiveness by Molecule Type, 2023 to 2033
Figure 198: MEA Market Attractiveness by Route of Administration , 2023 to 2033
Figure 199: MEA Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 200: MEA Market Attractiveness by Country, 2023 to 2033






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Atypical Antipsychotic Market
Warm Autoimmune Hemolytic Anemia (WAIHA) Treatment Market Analysis by Drug Class, Distribution Channel, and Region through 2035
Drug-Induced Immune Hemolytic Anemia Market - Demand & Forecast 2025 to 2035
Treatment-Resistant Hypertension Management Market Size and Share Forecast Outlook 2025 to 2035
Treatment-Resistant Depression Treatment Market Size and Share Forecast Outlook 2025 to 2035
Treatment Pumps Market Insights Growth & Demand Forecast 2025 to 2035
Pretreatment Coatings Market Size and Share Forecast Outlook 2025 to 2035
Air Treatment Ozone Generator Market Size and Share Forecast Outlook 2025 to 2035
CNS Treatment and Therapy Market Insights - Trends & Growth Forecast 2025 to 2035
Seed Treatment Materials Market Size and Share Forecast Outlook 2025 to 2035
Acne Treatment Solutions Market Size and Share Forecast Outlook 2025 to 2035
Scar Treatment Market Overview - Growth & Demand Forecast 2025 to 2035
Soil Treatment Chemicals Market
Water Treatment System Market Size and Share Forecast Outlook 2025 to 2035
Water Treatment Chemical Market Size and Share Forecast Outlook 2025 to 2035
Algae Treatment Chemical Market Forecast and Outlook 2025 to 2035
Water Treatment Market Size and Share Forecast Outlook 2025 to 2035
Water Treatment Ozone Generator Market Size and Share Forecast Outlook 2025 to 2035
Water Treatment Equipment Market Size and Share Forecast Outlook 2025 to 2035
Burns Treatment Market Overview – Growth, Demand & Forecast 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA