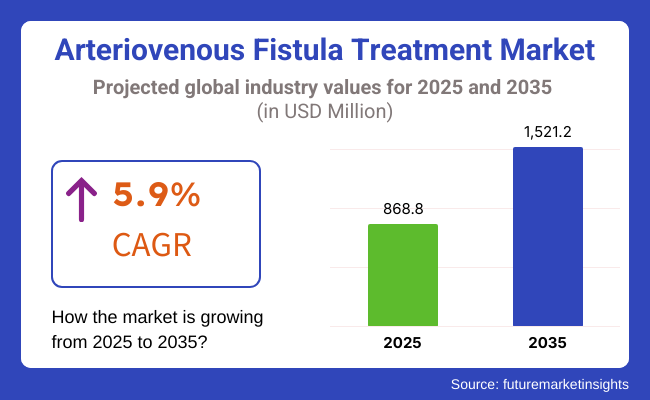
The market is projected to reach approximately USD 868.8 million in 2025 and expand to around USD 1,521.2 million by 2035, reflecting a compound annual growth rate (CAGR) of 5.9% over the forecast period.
The Arteriovenous Fistula will prosper as the majority of hemodialysis patients the world over rise due to rising ESRD incidence. The development in bioengineered grafts, drug-coated balloons, and another minimally invasive technique is helping make AVF therapy more efficient with long-term success and decreasing the risk of complications like stenosis and thrombosis.
Ultrasound-guided avf creation and endovascular methods are being increasingly utilized by physicians and clinicians to improve patient outcomes. North America and Europe have the lead as they have well-developed health care systems and favorable reimbursement policies. An uptick in demand is evident in the Asia Pacific and Latin American countries as health care access expands and more dialysis patients come in.
With innovations in biocompatible materials and regenerative medicine, AVF treatment will continue to improve, consequently offering patients better options with lesser chances of complications. However, these developments will be in place for a long time to make life easier and be the pulse for steady growth of the market for many years.
Market for arteriovenous fistula (AVF) treatment expanded rapidly over the last decade as increased ESRD populations, better medical treatment advances, and enhanced awareness of hemodialysis treatments. AVF is a preferred modality for most vascular access procedures among physicians due to its durability and decreased chances of infection over other procedures.
Bioengineered grafts, drug-eluting grafts, and minimally invasive approaches supplement these treatments on a whole new level. Robotic surgery, as introduced in the very early 2010s, had added precision to the whole new case of AVF creation, with avenues to decreased complications. Meanwhile, AI imaging systems solicit better real-time decisions from the hands of skilled surgeons, thus improving success rates and reducing human error.
Explore FMI!
Book a free demo
The treatment market for AVF in North America has gradually been boosting, primarily due to the increase in ESRD patients and the application of advanced vascular access systems by health systems within North America.
The country that moves ahead is the United States with a large number of medical devices' companies introducing modern technologies in the market along with government initiatives in promoting early CKD detection and treatment. In addition, the use of emerging minimally invasive techniques such as percutaneous fistula creation by physicians is expected to improve outcomes and reduce recovery time.
However, high procedure costs; insurance problems; and the shortage of well-qualified vascular surgeons are major obstacles to these patients and health care providers. Yet, many other positive aspects of artificial intelligence are influencing nephrology management of access for hemodialysis, while the wearable sensors perform real-time monitoring of AVF.
Meanwhile, researchers are busy developing new-generation drug-elution-grafts and infection-resistant devices that will improve long-term patency of AVFs and reduce complication rates.
The European treatment market for vascular access is undergoing a transformation with the increasing regulations, modern hemodialysis techniques, and more investments into vascular research thrusting the industry forward.
Germany, France, and the UK lead with comprehensive insurance coverage and an increasing number of advanced medical technology companies. Increasingly often, infected biocompatible vascular grafts resistant to infection and biocompatible vascular grafts that will minimize complications and improve patient management are being adopted by hospitals.
On the other hand, relatively variable models of healthcare funding in certain countries combined with a lag in regulatory approvals have thrown a barrier in front of the commercialization of some AVF treatments. On the bright side, robotic vascular surgeries and AI-based monitoring systems are reducing the risks associated with various treatments, making them safer and efficient.
Meanwhile, biotech firms and research institutions are working together in the bioengineering of vascular grafts that promise a better long-term outcome with fewer complications.
The treatment market for AVFs in Asia Pacific is booming due to the increase in chronic kidney diseases, the rise in health care investment, and awareness of vascular access. Among the countries experiencing demand for AVF creation and maintenance treatments due to rising figures of dialysis patients are China, India, and Japan. Governments are intervening by investing in better dialysis facilities, with the aim of making AVF treatments accessible to more beneficiaries.
However, the barriers remain: scarcity of vascular specialist surgeons, high costs, and inequitable access to healthcare may stunt patients in accessing necessary treatment. Hospitals are finding alternative low-cost techniques to create AVFs-an example is hybrid surgical-endovascular procedures.
On the other hand, researchers are studying possible biodegradable, regenerative vascular grafts within their works intended for Asian patients. Meanwhile, AI diagnostic equipment and robot-assisted AVF surgeries are making treatments more precise and effective, as well as patient-friendlier, across the region.
Challenges
Limited Awareness about AVF Maintenance and Postoperative Care is Emerging as the Significant Barrier for Market Growth
The AVF treatment market also encounters a number of real-life issues that adversely affect both the patients and medical professionals. The majority of the patients suffer from access failure, infection, or stenosis, which is usually followed by multiple procedures and increased healthcare expenditures. In cases of patients who have conditions such as diabetes or hypertension, AVF maturation becomes more complicated, and treatment success becomes difficult to attain.
Above all that, strict new regulations on new vascular access devices and sluggish reimbursement authorizations slow the delivery of improved therapies. In certain regions, a lack of qualified vascular specialists can leave patients unable to see AVF procedures in a timely fashion, jeopardizing their health in severe ways.
Another prime problem is that proper AVF care and postoperative care are not well-known. Most patients are faced with complications because they simply lack the knowledge. Filling this knowledge gap through enhanced patient education might be the single greatest step to enhance long-term results.
Opportunities
The Introduction of Nanotechnology-Based Coatings Emerging as The Significant Opportunity for Manufacturers
The fast-changing panorama of AVF treatment is opening avenues for better patient care. Increasingly, there are more talks among physicians and scientists around targeted treatment methods through patient-specific vascular grafts and machine learning-based fistula monitoring for improving outcomes. New advancements in endovascular therapy, such as creating percutaneous AVFs, are fast-tracking the procedures toward long-term success.
Dialysis chambers in most emerging countries increase their capacity to catch up with the demands, while doctors invest more in telemedicine to monitor their AVF patients. Meanwhile, researchers are working on bioengineered vascular grafts that have better compatibility with the body and even encourage natural healing.
There are also all kinds of cooperation among medical device companies and healthcare professionals pushed by innovation to bring new AVF management solutions and better to the market. Amazing changes such as nanotube coatings for grafts to reduce the risk of infection and clot formation are now making AVF procedures safer and more efficient for patients all around the world.
Growth in Bioengineered and Drug-Eluting Vascular Grafts
Physicians and scientists taking bold measures towards improving AVF therapy beyond bioengineered, drug-eluting vascular grafts as extending life of AVFs and reducing complications such as stenosis. Engineers are also designing tissue-engineered vascular grafts from bioresorbable or synthetic materials along with cells isolated from the patient's body. In this way, reduce clotting and infection risk, while the body constructs the vessel.
It also coats drugs in grafts like sirolimus and paclitaxel that can inhibit abnormal tissue formation, which accounted for most of the AVF failures. Besides, biodegradable stents and scaffold-based systems give temporary support yet will lose themselves over time and risk lowering long-term levels.
Thus, these advances point towards better AVF success rates, fewer repeat procedures, and better dialysis. As more people are in need of hemodialysis, these products of the new generation will keep people better while reducing treatment costs.
Advancements in Robotic-Assisted Vascular Surgery
Robotic-assisted surgery is changing the face of AVF procedures by rendering them more precise with less complication and long-term improvement. With robotic systems, the surgeons have the dexterity and precision to operate on tissues with less trauma, allowing for faster recovery for the patients.
AI-driven robotic systems go further by offering real-time imaging and navigation, allowing surgeons to locate blood vessels more precisely and facilitate improved intraoperative decision-making. Such accuracy decreases the fallibility of the AVFs should stenosis and thrombosis arise as complications.
An increasing utility of robots in the vascular access procedure, with the concurrent growth of robotic technology, assures safer surgeries, better patient outcomes, and lesser repeat interventions. This transformation is an exciting breakthrough in improving the care of hemodialysis patients globally.
| Market Shift | 2020 to 2024 |
|---|---|
| Regulatory Landscape | Compliance with safety and efficacy regulations for AVF surgical and minimally invasive procedures. |
| Technological Advancements | Adoption of imaging techniques and minimally invasive surgical approaches for AVF creation. |
| Consumer Demand | Increased awareness of ESRD and higher demand for effective, minimally invasive AVF treatments. |
| Market Growth Drivers | Rising ESRD prevalence and improvements in AVF creation procedures. |
| Sustainability | Initial adoption of eco-friendly manufacturing and reduced medical waste in AVF procedures. |
| Supply Chain Dynamics | Dependence on traditional distribution networks through hospitals and dialysis centers. |
| Market Shift | 2025 to 2035 |
|---|---|
| Regulatory Landscape | Stricter guidelines for non-surgical AVF creation and personalized treatment plans. |
| Technological Advancements | AI-driven diagnostics and real-time monitoring for optimized treatment plans. |
| Consumer Demand | Growing preference for non-surgical AVF creation and home-based dialysis solutions. |
| Market Growth Drivers | Expansion into emerging markets and continued innovation in non-invasive treatment options. |
| Sustainability | Widespread implementation of biodegradable materials and energy-efficient device production. |
| Supply Chain Dynamics | Growth in direct-to-consumer sales and telehealth-driven treatment planning. |
Market Outlook
This long-term hemodialysis access solution demand increases with the rapidly growing incidence of end-stage renal disease (ESRD). This accounts for consistent growth in the United States market for the treatment of AVFs. Technical improvements in bioengineered vascular grafts, drug-eluting grafts, and robotic-assisted laparoscopic surgeries improve the durability of AVFs, hence reducing their failure rate.
Additional market expansion is warranted by positive reimbursement policies and healthcare expenditure. Growing geriatric populations and high rates of prevalence of diabetes and hypertension provide further demand for AVF-related interventions. A growing rate of AMI intervention innovations is due to the participation of key players, research and development efforts, and regulatory approvals.
Market Growth Factors
Market Forecast
| Year | CAGR (2025 to 2035) |
|---|---|
| United States | 2.6% |
Market Outlook
Germany's market for arteriovenous fistula or AVF treatment is thriving, thanks to its well-structured healthcare system together with the rising prevalence of end-stage renal disease or ESRD and acceptance of novel vascular access solutions, all pushing demand. Increased emphasis on medical innovation within the jurisdiction has spurred the development of bioengineered grafts and robotic-assisted vascular intervention.
The magnanimous reimbursement schemes under statutory health insurance are consistently driving the market growth as well. The area of research collaboration among research institutes and medical device players is further escalating technological leaps. The rapidly aging population and the significant proportion of patients suffering from hypertension and diabetes are driving demand for AVF procedures.
Market Growth Factors
Market Forecast
| Year | CAGR (2025 to 2035) |
|---|---|
| Germany | 3.1% |
Market Outlook
The rapid growth of China's AVF treatment market is driven by rising ESRD and diabetes incidence which drives demand for advanced vascular access solutions. The Chinese government healthcare reforms that widen insurance coverage help access hemodialysis and the AVF procedure. Local players and global companies are investing in advanced technologies, such as drug-coated grafts and AI-powered robotic systems.
Market growth is also propelled by the increasing aging population and rapid urbanization. However, the gaps in urban versus rural health care quality persist. Increased investment in AVF technology and infrastructure will help with AVF accessibility and treatment efficacy.
Market Growth Factors
Market Forecast
| Year | CAGR (2025 to 2035) |
|---|---|
| China | 4.9% |
Market Outlook
There's a growing market for treatment of arteriovenous fistula (AVF) in India, which is mainly because of escalating numbers of patients suffering from End-Stage Renal Disease (ESRD) and an increasing population in need of dialysis. Government initiatives such as Ayushman Bharat are enhancing availability of services, bringing improvement in acceptance of AVF. Awareness and affordability of treatment are still major challenges in rural areas.
The expanding number of private health providers and foreign capital investment in nephrology care are factors driving the market. Bioengineered graft advancement as well as minimally invasive AVF surgery are increasing momentum while the introduction of new vascular access solutions is likely going to be facilitated by improved regulations.
Market Growth Factors
Market Forecast
| Year | CAGR (2025 to 2035) |
|---|---|
| India | 5.4% |
Market Outlook
The Brazilian AVF treatment market is expanding due to increasing ESRD burden and government programs to provide greater access for dialysis. Advance vascular access solution utilization is increasing due to private healthcare sector growth and an increasing investment in nephrology care.
Economic volatility and unequal access to healthcare among these obstacles, the increasing use of bioengineered and drug-eluting vascular grafts has positively impacted the outcome of AVF. Medical education and collaboration of local companies with international healthcare organizations are further fostering the development of robotic-assisted vascular techniques that could improve AVF patency rates and long-term dialysis outcomes.
Market Growth Factors
Market Forecast
| Year | CAGR (2025 to 2035) |
|---|---|
| Brazil | 4.3% |
Peripheral Arteriovenous Fistulas
Peripheral Arteriovenous Fistulae are the most common form of fistulae and are generally found in the extremities, arms, legs, and occasionally elsewhere due to trauma, congenital defect, or dialysis, constituting one of the most frequently occurring sites. These are abnormal arteriovenous communications that may cause symptoms: pain, swelling, and circulatory disturbances, thus making early detection and management very vital.
In addition, the increased incidence of dialysis-related AVFs, together with vascular injury, necessitates less invasive treatment modalities. In future will be the trends such as artificial intelligence used in vascular imaging to visualize early detection of AVFs, bioengineered AVF grafts to be used in dialysis patients, and in robot-assisted surgical treatment procedures, which will improve outcomes.
Dural Arteriovenous Fistulas (DAVFs)
They are conditions in the covering of the brain which are highly risky and must be treated urgently to prevent life-threatening conditions like intracranial hemorrhage, venous hypertension, and neurological disorders. Such conditions usually need management urgently. Otherwise, when life-threatening states develop, transcatheter embolization or surgical correction becomes mandatory.
With both traumatic and spontaneous DAVFs appearing and heightened awareness of neurovascular illness among physicians, the need for effective intervention continues to expand. Indeed, the future of treatment of DAVF will most likely include AI-driven cerebral angiography for intraoperative fistula mapping in real-time, the next generation of liquid embolic agents for more precise occlusion, and neuroprotectants to limit posttreatment complications.
Transcatheter Embolization
Transcatheter embolization is a preferred first line of treatment in AVFs, especially in the brain (DAVFs) or in peripheral sites. It is minimally invasive since coils or liquid embolic agents are injected to occlude them and relieve symptoms and complications through targeted abnormal blood flow. The increasing demand for this technique is caused by global trends in favor of endovascular over open surgical techniques and the increasing availability of more sophisticated embolic materials.
Coming improvements will include AI-guided catheter navigation, bioabsorbable embolic agents for temporal occlusion, and robotic embolization, and tomorrow's developments will make this technique even more precise and efficient.
Surgical Intervention
While minimally invasive techniques are the preferred choice, surgery is still an option for complex AVFs, particularly when embolization is not possible or unsuccessful. Surgical correction is typically reserved for high-flow peripheral fistulas, complex dural AVFs, and congenital vascular malformations.
Advances in surgical intervention have been made with the advent of microsurgical methods and the application of hybrid AVF treatment-surgery in combination with embolization. Robot-assisted surgery, artificial intelligence-based preoperative planning, and bioengineered vascular grafts to enhance the long-term success rate are poised to be called upon for AVF surgery in the future.
The market for arteriovenous fistula (AVF) treatment is growing swiftly with the increasing number of individuals in need of long-term dialysis from end-stage renal disease (ESRD). The need for good vascular access has boosted the demand, prompting businesses to innovate in areas such as stent grafts, balloon angioplasty, and bioengineered solutions.
The medical device makers, biotech companies, and healthcare professionals are working proactively towards defining the market through innovative technologies, advancing patient results, and success of the treatments.
Market Share Analysis by Company
| Company Name | Estimated Market Share (%) |
|---|---|
| Becton Dickinson & Company | 24.5% |
| Medtronic | 20.3% |
| B. Braun Melsungen AG | 14.2% |
| Cook Medical | 11.7% |
| Teleflex Incorporated | 9.8% |
| Others | 19.5% |
| Company Name | Key Offerings /Activities |
|---|---|
| Medtronic | Market leader offering advanced stent grafts, drug-coated balloons, and catheter-based AVF treatment solutions. |
| Becton, Dickinson and Company (BD) | Provides a comprehensive portfolio of vascular access products, including dialysis catheters and angioplasty balloons. |
| B. Braun Melsungen AG | Specializes in minimally invasive solutions for AVF maintenance, including drug-coated technologies. |
| Cook Medical | Develops innovative vascular grafts and bioengineered solutions for arteriovenous fistula repair. |
| Teleflex Incorporated | Offers interventional vascular devices and balloon angioplasty technologies to enhance AVF function. |
Key Company Insights
Medtronic (20.3%) A dominant player in the vascular access market, Medtronic provides state-of-the-art solutions for AVF creation and maintenance.
Becton, Dickinson and Company (24.5%) Known for its leadership in vascular access, BD offers a comprehensive range of devices for AVF treatment and maintenance.
B. Braun Melsungen AG (14.2%) A leading innovator in interventional treatments, Boston Scientific focuses on drug-coated balloons and catheter-based AVF solutions.
Cook Medical (11.7%) Specializing in durable and biocompatible graft solutions, Gore develops next-generation materials for AVF treatment.
Teleflex Incorporated (9.8%) A key provider of angioplasty and vascular intervention devices, Terumo supports AVF patients with minimally invasive solutions.
Other Key Players (19.5% Combined) Beyond the leading companies, several other manufacturers contribute significantly to the market, enhancing product diversity and technological advancements. These include:
The global arteriovenous fistula treatment industry is projected to witness CAGR of 5.9% between 2025 and 2035.
The global Arteriovenous Fistula Treatment industry stood at USD 820.5 million in 2024.
The global rare neurological disease treatment industry is anticipated to reach USD 1,521.29 million by 2035 end.
India is expected to show a CAGR of 5.4% in the assessment period.
The key players operating in the global Arteriovenous Fistula Treatment industry are Becton Dickinson & Company, Medtronic, B. Braun Melsungen Ag, Cook Medical, Teleflex Incorporated, Fresenius Medical Care AG & Co. KGaA and others.
Arteriovenous Fistulas, Dural, Peripheral, Pial or Cerebral and Other Fistula Types
Drugs, Transcatheter Embolization, Ultrasound-guided Compression and Surgery
North America, Latin America, Western Europe, Eastern Europe, East Asia, South Asia & Pacific, Middle East & Africa
Eyelid Scrub Market Analysis & Forecast by Product, Application and Region 2025 to 2035
Protein Diagnostics Market Share, Size and Forecast 2025 to 2035
CGRP Inhibitors Market Trends - Growth, Demand & Forecast 2025 to 2035
Indolent Systemic Mastocytosis treatment Market Insights: Size, Trends & Forecast 2025 to 2035
Intraoperative Fluorescence Imaging Market Report - Demand, Trends & Industry Forecast 2025 to 2035
Lung Cancer PCR Panel Market Trends, Growth, Demand & Forecast 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.