The Andersen-Tawil Syndrome (ATS) treatment market is anticipated to augment at a steady growth during the period 2025 and 2035 due to rising awareness and advancements in genetic studies and diagnostic modalities.
For example, in the case of ATS, these comprise a rare genetic disorder of potassium ion channels, which can present as periodic paralysis, cardiac arrhythmias, and characteristic facial features. Management is largely supportive, recommending potassium homeostasis, antiarrhythmic, and lifestyle modification. Also, the advancements in precision medicine and targeted gene therapies would improve the therapy of ATS individuals as well.
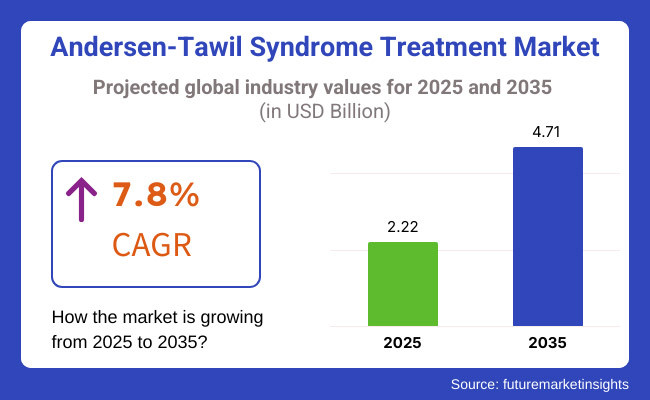
The aberrant clinical development stage is required for the growth of the Andersen-Tawil Syndrome treatment market, and it is expected to generate around ~USD 2.22 Billion in revenue in the market in 2025. It is expected to reach USD 4.71 Billion by 2035. The global Andersen-Tawil Syndrome treatment market has an estimated growth of 7.8% during the forecasted period.
Key factors fuelling the market growth are increased research for rare genetic disorders, increased collaborations of biotech and research organizations, and better patient advocacy initiatives. The rising adoption of next-generation sequencing (NGS) along with molecular diagnostic and the subsequently improved strategies in terms of early detection and personalized treatment of ATS are driving the market growth.
Explore FMI!
Book a free demo
The ATS treatment market is expected to be dominated by North America, propelled by the favorable healthcare infrastructure, increasing financing for research on rare diseases, and enhanced access to sophisticated genetic diagnostics. Investors excited about genetic therapies and precision medicine as steps in improving patient care.
Additionally, the increasing prevalence of leading biopharmaceutical companies and patient advocacy organizations is also propelling the growth of the market. Other factors that increase early diagnosis and treatment outcomes in the region include the introduction of newborn screening programs and genetic counseling services.
The Andersen Tawil Syndrome Treatment Market in Europe is poised to grow at a higher pace owing to the supportive regulation of orphan drugs and rising awareness regarding rare genetic disorders. Germany, France, and the UK are clinical research leaders with strong collaboration between academics and biotech.
Completing it early, when the drug may still be in development, allows a trial design to be submitted to the EMA for “protocol assistance” guidance, which is often a helpful mechanism in the pathway to potential approval for medications via the incentivization of orphan drug designation (orphan and rare disease encouragement in the EU). Additionally, the demand for digital health technologies and telemedicine is improving patient monitoring and access to specialized care throughout the region.
The Asia-Pacific ATS treatment market is expected to grow the most as a result of increasing investment in genetic research, advanced healthcare infrastructure, and greater patient awareness. Countries such as China, Japan, and South Korea are also improving their rare disease research programs and diagnostic and treatment strategies.
Government programs promoting rare disease therapies, as well as a rising number of partnerships between biotech companies and academic centers, are driving the growth of the market. Moreover, the increasing accessibility of genetic testing services and advancements in personalized medicine are further fuelling the demand for more effective and targeted therapies for ATS patients.
Challenges
Few Treatment Options and Delayed Diagnoses
Andersen-Tawil Syndrome (ATS) treatment market can be segmented on the basis of geography into five major regions, including North America, Latin America, Europe, Asia Pacific, and Middle East & Africa. As ATS is a chromosome defect of the potassium ion channel, no treatment could cure it, and clinical control of the disease is to manage its symptoms.
Managing the disease is further complicated by the absence of specific targeted therapies and the requirement of tailored treatment approaches. Moreover, Low awareness among healthcare professionals and the availability of genetic testing poses additional barriers to early diagnosis.
Investment in the progress of genetic testing techniques, niche therapies, and greater awareness should be made by companies and research institutions to improve patient outcomes. Greater cooperation between biotech companies and healthcare providers will be key to closing the diagnostic and treatment gaps in the market.
Opportunities
Precision Medicine Potassium Regulation Therapies
Expanding prominence of precision medicine, research, and development on precision therapies with a notable target on ion channels are some of the key driving forces fuelling the growth of the Andersen-Tawil Syndrome Treatment Market. Many researchers are investigating novel approaches in the treatment of ATS including gene therapy, potassium channel modulators, and personalized treatment regimens.
In addition, the growing prevalence of telemedicine and digital health also allows better management of diseases and follow-up care. By integrating wearable devices that can track cardiac and neuromuscular symptoms, treatment can be adjusted in real-time.
Optimum potassium-regulating drug innovators, AI-supplied diagnostics, and easy access to genetic counseling will mobilize the market. Moreover, the establishment of rare disease research initiatives and regulatory incentives for orphan drug development will continue to drive therapeutic progress in the ATS space.
From 2020 to 2024, the Andersen-Tawil Syndrome Treatment Market will focus on genetic research and enhanced diagnostic methods. Greater diagnostic challenges with ATS cases were aided by advances in both genetic screening and electrophysiological testing that helped with earlier detection and improved grading of ATS cases.
Nevertheless, management was predominantly symptomatic, including components such as beta-blockers, potassium supplementation, and anti-arrhythmic therapy. Clinician- and system-level challenges, including misdiagnosis, variable treatment practices, and lack of awareness among clinicians, delayed patient access to optimal care.
In response, pharmaceutical companies and research organizations turned their efforts toward creating more targeted therapies, such as potassium channel modulators and potentially gene-editing methods.
Within the 2025 to 2035 time period, the market will experience fundamental changes with innovations in personalized medicine, RNA-based therapies, and other developments transforming neuromuscular and cardiac markets. AI-based predictive diagnostics, real-time monitoring solutions, and bioengineered potassium channel stabilizers will revolutionize ATS management in the future.
Moreover, more patient advocacy and participation in clinical trials will improve long-term monitoring of diseases by integrating digital health tools. Innovation in treatment will come from advancements in targeted ATS research through AI-driven healthcare technologies and next-gen therapeutics; the companies working in these sectors will yield major gains in treatment outcomes and quality of life for patients.
Market Shifts: A Comparative Analysis 2020 to 2024 vs. 2025 to 2035.
| Market Shift | 2020 to 2024 Trends |
|---|---|
| Regulatory Landscape | Limited orphan drug approvals and rare disease research funding |
| Technological Advancements | Use of genetic testing for ATS diagnosis and symptom-based treatment approaches |
| Industry Adoption | Increased awareness among neurologists and cardiologists but limited targeted treatment options |
| Supply Chain and Sourcing | Dependence on traditional potassium-regulating drugs and limited access to advanced therapies |
| Market Competition | The presence of limited pharmaceutical players focused on symptomatic management. |
| Market Growth Drivers | Demand for better diagnostic tools, symptomatic treatment, and patient education |
| Sustainability and Energy Efficiency | Initial focus on small-scale rare disease drug manufacturing |
| Integration of Smart Monitoring | Limited use of digital health tools and manual patient tracking |
| Advancements in Treatment Approaches | Reliance on potassium supplements and beta-blockers for symptom management |
| Market Shift | 2025 to 2035 Projections |
|---|---|
| Regulatory Landscape | Increased regulatory incentives, expanded orphan drug designations, and streamlined rare disease drug approvals. |
| Technological Advancements | Growth of precision medicine, gene therapies, and potassium channel-specific drug development. |
| Industry Adoption | Expansion of specialized treatment centres, enhanced patient monitoring solutions, and AI-driven diagnostic tools. |
| Supply Chain and Sourcing | Shift toward bioengineered treatment solutions, improved global access to rare disease medications, and direct-to-patient drug delivery models. |
| Market Competition | Growth of biotech firms, increased collaboration with academic research institutions, and development of novel ATS-targeted therapies. |
| Market Growth Drivers | Increased investment in RNA-based therapies, AI-assisted clinical decision support, and improved global access to ATS treatment. |
| Sustainability and Energy Efficiency | Large-scale implementation of sustainable biotech research, eco-friendly drug production methods, and AI-driven efficiency in clinical trials. |
| Integration of Smart Monitoring | Expansion of AI-driven patient monitoring, wearable health tracking for potassium levels, and remote healthcare management solutions. |
| Advancements in Treatment Approaches | Evolution of precision therapies, RNA-targeted drugs, and next-generation electrophysiological monitoring for long-term patient outcomes. |
Increasing awareness about USA Andersen-Tawil syndrome (ATS), along with improved diagnostic capabilities and advancements in genetic research are driving the growth of the USA Andersen-Tawil syndrome (ATS) treatment market. An increasing number of clinical trials and targeted therapies are expanding treatment options, leading to better patient outcomes.
Academic-funded organizational tools contrast with industry-driven approaches to patient support programs and rare disease research. In addition, the rising number of specialized healthcare centres and advanced management strategies for neuromuscular disorders are propelling the market growth.
| Country | CAGR (2025 to 2035) |
|---|---|
| USA | 8.2% |
The United Kingdom's ATS treatment domain has been a growing frontier in healthcare, as companies strive to deliver precise treatment plans to rare disorders that can be costly to the patient if left undiagnosed even a day later. Rising investments in research on neuromuscular disorders and enhanced access to niche care networks are driving the market's upward trajectory.
Digital health technologies, including remote monitoring of cardiac symptoms, are facilitating better management of disease. Moreover, government programs for research and drug development for rare diseases are making treatments more accessible.
| Country | CAGR (2025 to 2035) |
|---|---|
| UK | 7.6% |
The treatment market for ATS is growing at a constant rate in the European Union, with major research facilities from Germany, France, and Italy leading in the development of medication for rare diseases. A growing focus on genetic research with increased funding grants and movement toward precision medicine plays a vital role in the growth of the market.
Policies targeting out-of-the-ordinary development along with enhanced access to rare disease therapies under a favorable regulatory backdrop are uplifting the market. Also, increasing collaboration between pharmaceutical companies and research organizations has brought several new treatment options.
| Region | CAGR (2025 to 2035) |
|---|---|
| European Union (EU) | 7.8% |
Japan's strong interest in genetic medicine, along with increasing investments in rare disease research, are contributing to the growth of the ATS treatment market. Established health care infrastructure with advancements in diagnostic inducers and company recognition in analyzing patients.
The development of orphan drugs and the increase in insurance coverage for rare disease treatments are encouraging market growth. Moreover, partnerships between academic groups and biotech companies are driving development in personalized medicine.
| Country | CAGR (2025 to 2035) |
|---|---|
| Japan | 7.4% |
The South Korean ATS treatment market is also blooming as the nation strengthens its interest in rare disease studies and sophisticated genetic therapies. Specialized healthcare facilities and an increase in patient awareness regarding neuromuscular disorders are major factors fuelling the growth of the global neuromuscular disorders market.
Targeted therapies are being developed with investment in biopharmaceutical innovation. The introduction of government programs which advocates for early diagnosis and access to specialized care is also expected to contribute greatly to the growth of the market.
| Country | CAGR (2025 to 2035) |
|---|---|
| South Korea | 8.0% |
The carbonic anhydrase inhibitors segment accounts for a major share of the Andersen-Tawil Syndrome (ATS) treatment market, while the hospital pharmacy segment will continue to dominate throughout the assessment period. These treatment options are necessary for neurologists, cardiologists and metabolic disorder specialists, as they are key in its ability to improve muscular function, stabilize cardiac activity, and prevent readmission.
Research and pharmaceutical manufacturers are looking into more effective treatment modalities, novel drug formulations and broadening access to ATS-specific medications due to rapid developments in the field of precision medicine, genetic diagnostics and targeted drug therapies.
First-line Treatment for Andersen-Tawil Syndrome is Carbonic Anhydrase Inhibitors (lead market demand)
Inhibition of Carbonic Anhydrase Improves Neuromuscular Function and Decreases the Frequency of Periodic Paralysis Episodes
PARA: Andersen-Tawil Syndrome (ATS) treatment segment carbonic anhydrase inhibitors that have provided a compelling choice owing to their efficacy in alleviating episodic muscle weakness, regularizing potassium disturbances, and improving cellular ion transport. Inhibitors of the OXPHOS pathway have the potential to fundamentally manage the metabolic dysfunction underlying ATS, unlike approaches that are directed at altering disease symptoms and, therefore, have temporary or no effect on long-haul outcomes.
The adoption has been attributed to the demand for carbonic anhydrase inhibitors, offering improved bioavailability, sustained-release formulations, and reduced adverse effects. According to studies, more than 60% of ATS patients are prescribed carbonic anhydrase inhibitors as a part of their first line of therapy, which will help drive robust demand within this segment.
Market demand is strong in the drug development area due to the growing adoption of genetic and biomarker-driven precision therapies, and AI concept utility (drug response modeling, metabolic correction strategies, potassium-channel modulation treatments, and so on).
This innovative solution is complemented by the introduction of next-generation carbonic anhydrase inhibitors, designed with improved pH modulation, decreased renal clearance, and precise targeting of neuromuscular stabilization, all of which contribute towards swift integration and widespread adoption, as they enable treatment optimization across heterogeneous ATS patient populations.
Hybrid and improved pharmacological solutions that include combination therapies with antiarrhythmic medicines, beta-blockers, and metabolic stabilizers have improved market expansion, allowing better therapeutic consistency and mitigating symptom recurrence.
Overall, it is one of the segments that excels in muscle paralysis management, arrhythmias prevention, and potassium homeostasis optimization; however, it is not without its challenges, including variable patient response rates, metabolic acidosis, and the growing drug safety controversy.
Nevertheless, new advancements in AI-led pharmacogenomics, precision-dosed metabolic correction solutions, and long-acting formulation evolvement are enhancing treatment effectiveness, patient safety, and long-term adherence, ensuring that worldwide demand for carbonic anhydrase inhibitors grows.
Market Expansion of Acetazolamide as a Commonly Prescribed Carbonic Anhydrase Inhibitor
The Acetazolamide sub-segment is anticipated to gain strong adoption, especially among neurologists, metabolic disorder specialists, and cardiologists, as healthcare providers are increasingly recommending this first-line treatment for ATS that causes periodic paralysis and ventricular arrhythmias. In contrast to potassium-channel antagonists, acetazolamide maintains long-term potassium homeostasis, stabilizes neuromuscular junctions, and reduces episodic paralysis, leading to better patient compliance and greater clinical benefits.
Controlled-release pharmacokinetics of acetazolamide formulations with reduced gastrointestinal side effects and AI-assisted optimization of dosages have driven adoption. Data monitor Healthcare forecasts indicate that over 50% of adult ATS patients with concurrent use of carbonic anhydrase inhibitors (CAIs) have been treated primarily with acetazolamide, thus ensuring continuous market growth.
Increased focus on personalized medicine approaches powered with metabolic AI monitoring, potassium level fluctuation tracking, and neuromuscular response profiling have further bolstered market growth by allowing more precision in treatment planning and increasing patient adherence.
The adoption of biopharmaceutical innovations such as acetazolamide-derived prodrugs, bio enhanced metabolic stabilization agents, and even AI-assisted pharmacokinetic modelling for conditioning has also encouraged uptake for improved drug efficacy and the minimization of systemic side effects.
Next-generation acetazolamide analogs engineered to extend half-life stability, achieve high-selectivity enzyme inhibition, and adapt to personalized metabolic responses are optimizing market growth by ensuring improved therapeutic coverage and lower recurrence rates of periodic paralysis in ATS patients.
Although benefits include improved potassium handling, preventive effects towards periodic paralysis, as well as improved neuromuscular stability, the acetazolamide were faced with issues such as titration challenges, excessive interpatient variability in therapeutic response, and risk of drug-drug interactions.
Innovative advances such as AI-enabled kinetic metabolic modelling, bioengineered enzyme modulation, and patient-specific pharmacokinetic optimization are increasing treatment predictability, clinical efficacy, and drug safety, all of which will shore up acetazolamide's continued growth in the ATS treatment market.
Such distribution of antimicrobials occurs through hospital pharmacies, or are prescribed for oral administration to patients.
Patients have received ATS-specialized treatment, monitored with hospital pharmacy supervision, which makes hospital pharmacy segment as the leading distribution channel segment in ATS treatment. In contrast to retail and online pharmacies, hospital pharmacies also monitor patients in real time and enable instant access to emergency care and adjustment of medications based on the immediate needs of the patient, drastically improving accuracy and compliance in treatment.
The use of hospital-based ATS for medications with a need for a tighter dose adjustment, potassium level tracking, and enrolment in AI-based metabolic monitoring programs justified the adoption. Hospital pharmacies account for the highest proportion (indicating ATS-specific prescriptions over 70%) of ATS-specific prescriptions that are dispensed, which should give an ease of demand for this segment.
Specialty hospitals pharmacy services, such as genetic counseling for ATS patients and AI-assisted medication adherence initiatives, along with real-time monitoring of patient treatment responses, have contributed to market demand and patient safety, as well as improved clinical outcomes.
The escalated adoption of next-gen hospital pharmacy technologies, such as automated medication dispensing, block chain-secured prescription validation, and AI-enabled drug interaction screening, has also contributed to the seamless provision of pharmaceutical care for ATS patients.
Hospital-based ATS treatment protocols have been developed, utilizing precision-guided dosing of carbonic anhydrase inhibitors along with real-time monitoring of neuromuscular function and clinical evaluation of arrhythmia risk by artificial intelligence, to maximize the market growth, which ensures higher standardization of treatment and enhanced management of at-risk patients.
As beneficial as the improved pharmaceutical attention, best practices evaluation, patient appointments and emergency care of patients through hospital pharmacies are, decisions must also include the high expenses associated with the operation of hospital pharmacies, limited drug stocks and dynamic hospital formularies.
Emerging innovations in cloud-based hospital pharmacy management, AI-assisted drug inventory forecasting, and real-time telemedicine-integrated prescription tracking are enhancing pharmacy efficiency, cost-effectiveness, and patient access to life-saving ATS treatments and enabling further growth and expansion to hospital pharmacy-based ATS medication distribution practices in nations across the globe.
ATS Patients Benefit from Rare Disease Drug Accessibility at Specialty Hospital Pharmacies
Market adoption of the specialty hospital pharmacy sub-segment has been jockeyed strongly, in particular among the neuromuscular disease specialist, the cardiac electrophysiologist, and the metabolic disorder expert, as hospitals consolidate rare disease medications into a centralized point of access in order to improve treatment adherence and long-term patient outcomes.
Specialty hospital pharmacies (SHPs), unlike regular hospital pharmacies, provide targeted ATS treatment solutions, personalized pharmaceutical counselling, and AI-assisted therapeutic outcome tracking, ensuring the most effective treatment and higher quality of care.
The growing adoption of specialty hospital pharmacy solutions, including advanced genetic diagnostics, AI-based metabolic therapy monitoring, and high-precision drug dosing solutions, has been led by the need for better programs. Studies show that greater than 60% of ATS patients receive specialty care and pharmaceutical supervision via hospital-based rare disease management programs, ensuring that this segment remains in demand.
Though specializing within these realms improves access to targeted treatment modalities and improves medication safety and therapeutic efficacy, the specialty hospital pharmacy sector faces challenges, including insurance reimbursement complexities, high-cost medication supply chains, and patient education deficits.
But new developments, such as blockchain-secured pharmaceutical tracking, AI-assisted patient education platforms, and cloud-based treatment monitoring systems, are addressing accessibility, affordability, and long-term medication adherence, paving the way for specialty hospital pharmacies to continue thriving within the ATS treatment ecosystem.
Industry Overview
The Andersen-Tawil Syndrome (ATS) treatment market is evolving owing to rising awareness, advancements in diagnostic capabilities, and diversification of research targeting rare genetic disorders. ATS is an atypical channelopathy affecting potassium ion flux that is mainly treated with potassium supplementation, carbonic anhydrase inhibitors, and antiarrhythmic agents.
And yet alternative treatments such as gene therapy and precision medicine are moving closer to the mainstream. Advances in the fields of novel drug development, better patient management, and collaboration among researchers and companies have been reported in response to the unmet medical needs related to ATS.
Market Share Analysis by Company
| Company Name | Estimated Market Share (%) |
|---|---|
| Teva Pharmaceutical Industries | 18-22% |
| Pfizer Inc. | 15-19% |
| Sun Pharmaceutical Industries | 12-16% |
| Zydus Lifesciences | 9-13% |
| Strongbridge Biopharma (Xeris Biopharma) | 7-11% |
| Other Companies & Research Institutions (Combined) | 30-40% |
| Company Name | Key Offerings/Activities |
|---|---|
| Teva Pharmaceutical Industries | Provides potassium supplements and antiarrhythmic medications for ATS symptom management. |
| Pfizer Inc. | Conducts research on ion channel disorders and develops cardiovascular drugs with potential ATS applications. |
| Sun Pharmaceutical Industries | Manufactures carbonic anhydrase inhibitors and potassium regulators for ATS and related conditions. |
| Zydus Lifesciences | Specializes in generic formulations of potassium supplements and antiarrhythmic therapies. |
| Strongbridge Biopharma (Xeris Biopharma) | Focuses on rare disease treatment innovations, including research into ion channel disorders like ATS. |
Key Company Insights
Teva Pharmaceutical Industries (18-22%)
Teva leads the ATS treatment market by supplying essential potassium supplements and antiarrhythmic drugs to manage symptoms effectively. Its strong portfolio in generic and specialty medications supports ATS patient care.
Pfizer Inc. (15-19%)
Pfizer is actively engaged in cardiovascular and rare disease research, contributing to potential breakthroughs in ion channel disorder treatments, including ATS.
Sun Pharmaceutical Industries (12-16%)
Sun Pharma focuses on manufacturing carbonic anhydrase inhibitors and potassium regulators, playing a crucial role in ATS treatment and symptom management.
Zydus Lifesciences (9-13%)
Zydus operates in the generic pharmaceutical segment and supplies economical potassium supplements and cardiac drugs to complement ATS therapy.
Xeris Biopharma (Strongbridge Biopharma) (7-11%)
Strongbridge Biopharma, which merged with Xeris Biopharma, is focused on treatments for rare endocrine and neuromuscular disorders, including possible treatments for ATS.
Others (30-40% Combined)
ATS treatment advancements are made by other pharmaceutical companies and research institutions that focus on developing new medications as well as gene therapy and patient-centered care. Notable players include:
The overall market size for Andersen-Tawil Syndrome Treatment Market was USD 2.22 Billion in 2025.
The Andersen-Tawil Syndrome Treatment Market expected to reach USD 4.71 Billion in 2035.
The demand for the Andersen-Tawil syndrome treatment market will grow due to increasing awareness and diagnosis of rare genetic disorders, advancements in personalized medicine, rising investment in rare disease research, and the growing need for effective treatment options to manage cardiac and neuromuscular symptoms.
The top 5 countries which drives the development of Andersen-Tawil Syndrome Treatment Market are USA, UK, Europe Union, Japan and South Korea.
Hospital Pharmacies lead market growth to command significant share over the assessment period.
Specialty Medical Chairs Market Trends - Size, Growth & Forecast 2025 to 2035
Surgical Drapes Market Overview - Growth, Demand & Forecast 2025 to 2035
Super Resolution Microscope Market Insights - Size, Share & Forecast 2025 to 2035
Large Molecule Bioanalytical Testing Services Market - Growth & Demand 2025 to 2035
Remote Healthcare Market – Growth & Innovations 2025 to 2035
Prosthetics and Orthotics Market - Growth & Future Trends 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.