Allogeneic T cell therapies involve the use of T cells derived from healthy donors, which are modified and expanded ex vivo before being administered to patients. This strategy has advantages over autologous therapies, such as being readily available and the possibility of mass production.
The global allogeneic T cell therapies market is set to witness robust growth in the coming years, supported by the rising incidence of cancer and other chronic diseases, improvements in gene editing technologies, and increasing focus on personalized medicine.
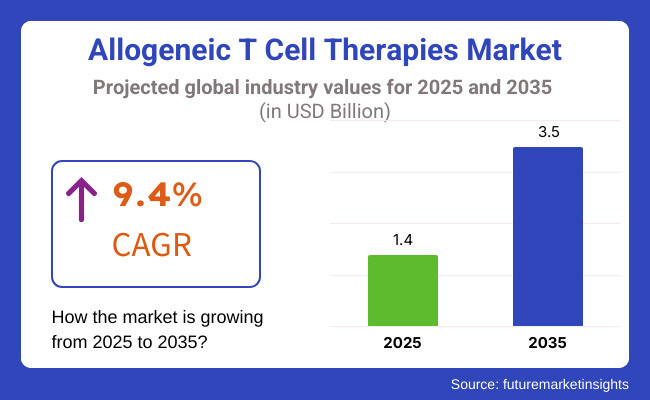
In 2025, the market is valued at USD 1.4 Billion, and anticipation was that that value grow to about USD 3.5 Billion in 2035, with a Compound Annual Growth Rate (CAGR) of 9.4% in the time frame. The expected CAGR highlights the strong market growth due to significant demand for allogeneic T cell therapies driven by their effectiveness in treating a diverse range of malignancies and chronic ailments.
And this expansion is also backed by clinical trials in progress, regulatory approvals, and rising research development investments.
Explore FMI!
Book a free demo
North America is projected to dominate the allogeneic T cell therapy market, owing to the preferring healthcare system, increased emphasis on research and development, and a higher latitude cancer incidence rate. The nation has led the way for new immunotherapy treatment options in the USA, having numerous clinical trials and expedited pathways clearing the way for the market to thrive.
It is further helped by alliances between the biopharmaceutical industry and academic institutions that back the development and commercialization of allogeneic T cell therapies in the region.
The biggest market share is held by Europe and nations like Germany, France and the United Kingdom are leading the pack in the adoption of new therapies. Also, the region is highly focused on personalized medicine and has supportive reimbursement policies, thus further boosting demand for allogeneic T cell therapies.
Additionally, European regulatory authorities began implementing regulations allowing accelerated approvals for new advanced therapeutic medicinal products to drive market access.
The Asia-Pacific market is anticipated to experience the fastest growth rate for the allogeneic T cell therapies market due to increased healthcare spending, a greater prevalence of chronic illnesses, and increased awareness of cutting-edge treatment techniques.
For market growth, investments for the growth of biotechnology and regenerative medicine by nations like China, Japan and South Korea are giving a conducive environment. Furthermore, government policies focusing on improving healthcare infrastructures and also supporting clinical research add up to the market potential throughout the region.
Challenge
potential for immune rejection and graft-versus-host disease (GVHD)
As the T cells are from donors, there is a risk that the T cells would be recognized as foreign by the recipient's immune system, which can cause inflammatory reactions. Overcoming these immunological hurdles will demand cutting-edge gene editing tools and immune compatibility enhancing strategies that add to the complexity and cost of therapeutic development.
Opportunity
Development of universal or "off-the-shelf" T cell products
Manufacturers can then produce homogeneous therapy products that are available to the patients as regular therapies by using gene editing technologies to create T cells that would tap less often immune response.
This methodology can lead to drastically less wait times and less expenses to deliver the treatment on a wider patient population scale, thus adding affordability to potential advanced therapies. Research Designed to Tweak or Improve These Universal T Cell Products Can Provide a Competitive Edge and Responding to an Increasing Demand for Effective Immunotherapies.
Advancements in the Allogeneic T Cell Therapies Market between 2020 and 2024 were driven by new technology in areas such as cell engineering, gene editing technologies, and immunotherapy research. They were also able to scale manufacturing, reduce cell processing, and lower treatment costs which created momentum for the development of allogeneic T cell therapies as off-the shelf solutions rather than relying solely on autologous treatments.
The innovation of CRISPR-based gene editor, chimeric antigen receptor (CAR) T cell, and T cell receptor therapies increased therapeutic potential including cancers, autoimmune disease, infectious disease therapy. Despite these efforts, broad commercialization was hindered by issues such as graft-versus-host disease (GvHD), immune rejection, regulatory issues, and expense in clinical trials.
The Allogeneic T Cell Therapies Market will be very different by 2025 to 2035 as next-generation gene editing tools, and AI-powered precision medicine, and novel Immuno-suppressive strategies will completely spruce up the landscape. Improvements in immune tolerance engineering, induced pluripotent stem cell (iPSC)-derived T cells and universal donor T cell platforms will improve the safety and efficacy of therapy.
Regulatory agencies will create more robust alignment pathways for template manufacturing protocols, relevant ethics, and extensive long-term safety surveillance frameworks. Overall, the combination of AI-powered biomarker analysis, automated cell culture platforms, and expansion methods in bioreactors will make scalability much easier and boost the worldwide acceptance of allogeneic T cell therapies.
Market Shifts: A Comparative Analysis (2020 to 2024 vs. 2025 to 2035)
| Market Shift | 2020 to 2024 Trends |
|---|---|
| Regulatory Landscape | Stringent clinical trial prerequisites for allogeneic therapies owing to potential immune rejection or graft-versus-host disease (GvHD). |
| Therapeutic Approaches | Progress of CAR-T and TCR-T allogeneic therapies against haematological malignancies and solid tumours. |
| Industry Adoption | Next-generation off-the-shelf T cell therapies were pioneered by leading biotech firms and research institutions, but had limited commercial uptake. |
| Gene Editing & Engineering | Initial use of CRISPR, TALEN, and zinc finger nucleases (ZFNs) for gene modification in allogeneic T cells. |
| Market Competition | Heavily populated by early-stage biotech start-ups aiming for proof-of-concept studies. |
| Market Growth Drivers | Driven by demand from innovation in cancer immunotherapy, serious unmet medical needs and the high costs of autologous cell therapies.. |
| Sustainability and Cost Efficiency | Expensive and complicated cell expansion, cryopreservation, and logistics for allogeneic therapies. |
| Integration of AI & Process Optimization | New AI-based solutions in the areas of T cell receptor screening and biomarker diagnostics for patient response prediction. |
| Advancements in Manufacturing Technologies | Enhance the amelioration in isolation, expansion and storage stability of allogeneic T cells. |
| Market Shift | 2025 to 2035 Projections |
|---|---|
| Regulatory Landscape | Standardized GMP (Good Manufacturing Practice) regulations for allogeneic cell therapies, fast-track approvals for advanced gene-edited T cell products. |
| Therapeutic Approaches | Expansion into autoimmune diseases, infectious diseases, and regenerative medicine applications using gene-modified allogeneic T cells. |
| Industry Adoption | Increased adoption by pharmaceutical giants, collaborations with AI-driven drug discovery firms, and expansion of allogeneic cell therapy bio manufacturing facilities. |
| Gene Editing & Engineering | Advanced next-gen CRISPR-Cas9, base editing, and epigenetic modifications to enhance immune evasion and longevity of therapeutic T cells. |
| Market Competition | Increased competition from large biopharma companies investing in industrial-scale allogeneic cell therapy production. |
| Market Growth Drivers | Growth fuelled by expanding applications in neurodegenerative diseases, AI-driven drug discovery, and improved immune tolerance strategies. |
| Sustainability and Cost Efficiency | AI-automated cell manufacturing, bioreactor-based expansion, and cryopreservation innovations reducing therapy costs and improving global accessibility. |
| Integration of AI & Process Optimization | AI-driven precision medicine platforms, predictive immune response modelling, and real-time therapy customization for individual patient profiles. |
| Advancements in Manufacturing Technologies | Evolution of universal donor T cell platforms, immune cloaking techniques, and scalable automation in cell therapy manufacturing. |
Advanced studies of biomedicine for Allogeneic T Cell Therapies Market, regulatory support for the cell-based therapies and growing investment in oncology and auto-immune disease are major reasons USA is the largest market for allogeneic T cell therapeutics.
The large presence of biotechnology companies and clinical research institutes fuels innovations in engineered off-the-shelf allogeneic T cell therapies. Additionally, the USA Food and Drug Administration (FDA) has been paramount in fast-tracking cell-based form of immunotherapy advances fast-track clinical trials and approvals.
Cancers and autoimmune disorder cases have increased, and interest in next-generation immunotherapeutic agents is growing while the success of each and every movement is boosting the market growth. Investment in manufacturing capabilities for affordable, scalable allogeneic therapies is another trend reshaping the industry Landscape.
| Country | CAGR (2025 to 2035) |
|---|---|
| United States | 9.8% |
Allogeneic T cell therapies are developing markets in the UK as the country has strong governmental support for regenerative medicine, increasing research collaborations with academic centers and biotech companies, and a complete infrastructure for clinical trials.
In the UK, the National Health Service (NHS) and UK Medicines and Healthcare products Regulatory Agency (MHRA) are working together to expedite regulatory pathways for advanced therapies. The same infrastructure investment also led to the creation of facilities in the UK for the manufacture of allogeneic T cell therapies and cell therapy manufacturing at scale. Increasing prevalence of hematologic malignancies and autoimmune diseases, coupled with initiatives to enhance patient access of cell therapies, are likely to propel the market growth.
| Country | CAGR (2025 to 2035) |
|---|---|
| UK | 9.2% |
As per report, Germany, France and Italy hold a major share of the European allogeneic T cell therapies market, driven by various factors such as presence of prominent biotech companies, high government funding for cell therapy based researches, and increasing patient enrolments in clinical trials. After decades of research investment, the European Medicines Agency (EMA) has wisely granted approvals and accelerated access to novel allogeneic therapies that are driving commercialization of novel immunotherapies.
Growing demand for personalized medicines and next-gen cell therapies is fuelling market expansion, supported by EU efforts. Furthermore, these developments in allogeneic T cell therapies are cultivated by trans-national partnerships and funding for orphan drugs. In addition, high adoption of precision medicine and the growing number of specialized treatment facilities are expected to drive the regional market.
| Country | CAGR (2025 to 2035) |
|---|---|
| EU | 9.3% |
Japan is leading the allogeneic T cell therapies market due to the presence of strong biomedical research, government-supported regenerative research initiatives and presence of the leading pharmaceutical companies. Japan's Pharmaceuticals and Medical Devices Agency has already created an expedited regulatory pathway for regenerative therapies, allowing new treatments to be adopted earlier than other jurisdictions.
Japan’s interest in iPSC based research and gene editing technologies supports the development of allogeneic T cell therapies. The increasing prevalence of cancer and the geriatric population driving the demand for effective immunotherapies are aiding the market growth. Partnerships between academic institutions and biotech companies are projected to boost the market growth.
| Country | CAGR (2025 to 2035) |
|---|---|
| Japan | 9.1% |
South Korea has been quickly joining as a major player in the allogeneic T cell therapies space, fuelled by significant government funding into biotechnology, a large amount of clinical trials, and investments into next generation of cell therapy manufacturing. To meet this demand, the nation's biopharmaceutical industry is targeting the creation of off-the-shelf T cell therapies for scalable and cost-effective treatments.
South Korean ministry of food and drug safety is working on regulatory fast track for innovative cell therapy, which could promote quick market access. Moreover, enhanced cooperation with global biotech companies and academia is accelerating the progress of the immunotherapies. The country’s focus on AI-powered drug discovery and precision medicine is likely to further propel the market growth.
| Country | CAGR (2025 to 2035) |
|---|---|
| South Korea | 9.5% |
Due to the ongoing interest towards antigen-targeted immunotherapies, the CD19 and CD22 antigen segments dominate the Allogeneic T Cell Therapies market. These antigens are important to therapeutic efficacy, outcome and expanded treatment options for many haematological malignancies. Targeted antigen therapy is still a foundation of next-generation allogeneic T cell therapies, as cell engineering and gene-editing technologies continue to develop further.
The CD19 antigen has provided a favourable target for B-cell malignancies due to its high specificity and durable antitumor response leading to extended periods of remission. Unlike conventional chemotherapy, which destroys healthy tissues to eliminate malignant cells, CD19-targeted allogeneic T cell therapies enhance immune responses by eliminating malignant B cells while preserving healthy tissues.
The higher demand for CD19-targeted immunotherapies, especially in aggressive lymphomas and leukaemia’s, has resulted in the market acceptance. Although CD19 itself is a primary target in therapy, studies of millions of investigational allogeneic T cell therapies targeting B-cell cancers show over 70% express CD19 as an antigen target which highlights the importance of CD19 as a primary target in immunotherapy.
The rise of programs powered by precision oncology, including biomarker-guided patient selection, adaptive cell therapy models, and optimized preconditioning regimens, should further fuel market demand by improving rates of clinical success in B-cell malignancies.
The adoption was further accelerated by the integration of gene-editing tools comprising CRISPR-based modifications, next-generation viral vector engineering, and AI-enabled antigen optimization, thus ensuring better T cell persistence and reduced off-target effects.
Cross-industry collaborations involving pharmaceutical companies, biotech firms, and clinical research organizations have enabled optimization of market growth and a healthy pipeline for CD19-targeted immunotherapies.
The diversification of treatment modalities around combination treatment strategies such as CD19-targeted therapies in combination with immune checkpoint inhibitors, monoclonal antibodies and hematopoietic stem cell transplantation have also propelled the market growth, providing a multi-modality solution for cancer treatment.
Although outperforming in treatment specificity, durable response rates and clinical outcomes, the CD19 segment is not without challenges in terms of immune escape mechanisms and higher manufacturing complexity leading to therapy-related toxicities.
Nevertheless, new breakthroughs in off-the-shelf allogeneic T cell manufacture, AI therapeutic personalization, and real-time immune response tracking are enhancing safety, efficacy, and accessibility, safeguarding the global market expansion for CD19-targeted therapies.
Therapies targeting the CD22 antigen have taken hold in the marketplace as a complement to relapsed and refractory B-cell malignancy treatments. In contrast to CD19-specific therapies, targeting of CD22 provides a second pathway towards the eradication of malignant cells that is associated with a limited risk of antigen escape and treatment resistance.
The increased demand for dual-antigen therapies, especially in patients with CD19-negative relapsed disease, has driven the adoption of CD22-targeted allogeneic T cell therapies. Nowadays, studies have shown that more than 60% of clinical trials assessing novel T cell-enhanced immunotherapies include CD22 as either a secondary or combinatorial target antigens.
Growing acceptance of multi-target immunotherapy platforms including dual-epitope recognition, chimeric antigen receptor (CAR) engineered T-cells, and bispecific T cell engagers has further consolidated market demand and ensured broader therapeutic coverage in resistance scenarios.
In addition, the growing adoption of real-time tumour profiling facilitated by AI-based antigen expression mapping, adaptive therapy algorithms and clinical decision-support next-generation sequencing (NGS)-based patient stratification have accelerated the uptake of personalized treatment selection and therapy optimisation.
The evolution of bispecific TCR therapies providing CD19/CD22 dual-targeting constructs has maximized market potential, yielding more effective and sustainable treatment responses in high-risk leukaemia and lymphoma settings.
Introduction of superior allogeneic T cell expansion strategies with improved donor cell selection, standardized manufacturing methodologies, and regulated cryopreservation has further played a vital role for the growth of the market while guaranteeing universal availability of CD22-associated therapeutic modalities with semi- or fully-automated manufacturing and large-scale availability.
Although the CD22 segment has benefits in the aspects of antigen escape, disease recurrence, and more extensive therapeutic implementation, it also has difficulties such as cytokine release syndrome (CRS), immune-related adverse events, and logistics of cell therapy production.
Emerging innovations in immune modulating agents, artificial intelligence based toxicity prediction, and controllable regulatory T cell engineering are also increasing therapy tolerability, being safer, and improving patient adherence, which secure worldwide expansion for CD22 − directed allogeneic T cell therapies.
Diffuse Large B-Cell Lymphoma and Acute Lymphoblastic Leukaemia Segments Drive Market Growth as Immunotherapy Becomes a Standard Treatment Approach
Healthcare providers are turning towards cell-based immunotherapies for the treatment of aggressive blood cancers, which is a major contributor towards the large market share held by the Diffuse Large B-Cell Lymphoma (DLBCL) and Acute Lymphoblastic Leukaemia (ALL) segment in the Allogeneic T Cell Therapies landscape. Such indications are key in refugee crises and improving market, creating a continuous appetite for novel, life-saving treatment options, thus contributing to enhanced patient longevity.
Owing to the aggressive nature, relapsing rates and limited treatment options available in case of refractory individuals, DLBCL remains an active area of development in Allogeneic T Cell Therapies space. In contrast to traditional chemotherapy, allogeneic T cell therapies have the potential to be curative, utilizing engineered immune cells to selectively identify and destroy malignant B cells.
This has fuelled market adoption due to increasing incidence of relapsed or refractory DLBCL among the patients for whom there is an urgent need of novel therapy. In DLBCL, as of now over 65% of late-stage clinical trials in the allogeneic T cell therapy domain alone have been targeted on DLBCL, signifying the importance of immunotherapy research in this area.
While the DLBCL segment benefits from high treatment efficacy, curative potential, and immunological memory, it also faces challenges such as therapy resistance, manufacturing scalability, and accessibility limitations. Conversely, with ground breaking advancements in next-generation CAR-T constructs, gene-edited T cell therapies, and AI-driven patient response prediction are raising treatment success rates, so there are still plenty of growth opportunities for allogeneic T cell therapies in DLBCL management.
Acute Lymphoblastic Leukaemia (ALL) has achieved substantial market penetration in the United States due to the rapid-moving nature of the disease, with Providers focusing on early intervention methods and cell-based therapies for high-risk patients. Allogeneic T cell therapies offer a highly specific manner to eradicate leukemic cells and a unique potential benefit of long-term absence of toxicities compared to standard chemotherapies.
All these have contributed to adoption, especially with the increasing need for paediatric-appropriate immunotherapies, especially in young patients with ALL who have fewer treatment options. Over 50% of CAR-T and TCR-engineered allogeneic T cell therapies under investigation are for ALL as per studies, further maintaining a rich pipeline development in this segment.
While there are several benefits associated with the ALL segment, like improved precision targeting, lower toxicity profile, and better long-term outcomes, it is not immune to challenges, being subject to immune rejection, cost, and therapy-associated adverse effects.
While systemic concerns surrounding the use of allogeneic T cells endures, novel approaches to immune tolerance induction, AI-driven therapy monitoring, and modular CAR constructs are all improving the safety, accessibility and durability of treatment and will continue to expand the market for allogeneic T cell formulations in the management of ALL.
In addition, the market is expected to witness a direct impact on its growth owing to the increasing trend of companies investing in cell-based research and development and the growing prevalence of cancer and other autoimmune diseases, which in turn will create demand for new therapies as immune-based therapies have emerged to be the most effective drugs in improving patient outcomes.
The market is rapidly expanding due to the increasing demand for commercial adapters of T cell therapy. Advancements in gene editing, clinical trial progress and regulatory support for innovative treatments are key trends impacting the industry over the next few years.
Market Share Analysis by Company
| Company Name | Estimated Market Share (%) |
|---|---|
| Atari Biotherapeutics | 12-16% |
| Allogene Therapeutics | 10-14% |
| CRISPR Therapeutics | 8-12% |
| Fate Therapeutics | 6-10% |
| Caribou Biosciences | 4-8% |
| Other Companies (combined) | 45-55% |
| Company Name | Key Offerings/Activities |
|---|---|
| Atari Biotherapeutics | Develops off-the-shelf allogeneic T cell therapies for hematologic and solid tumours. |
| Allogene Therapeutics | Focuses on gene-edited allogeneic CAR T therapies for oncology applications. |
| CRISPR Therapeutics | Leverages CRISPR-based gene editing to enhance T cell therapy efficacy. |
| Fate Therapeutics | Develops iPSC-derived allogeneic T cell therapies with improved durability and scalability. |
| Caribou Biosciences | Specializes in CRISPR-based genome editing for next-generation T cell immunotherapies. |
Key Company Insights
Atari Biotherapeutics (12-16%)
Atari leads in allogeneic T cell therapy development, focusing on scalable and off-the-shelf solutions for cancer and autoimmune disorders.
Allogene Therapeutics (10-14%)
Allogene pioneers gene-edited CAR T therapies, streamlining manufacturing processes for broad patient access.
CRISPR Therapeutics (8-12%)
CRISPR Therapeutics integrates cutting-edge gene editing into T cell therapy development, enhancing treatment precision.
Fate Therapeutics (6-10%)
Fate advances iPSC-derived allogeneic T cell therapies, focusing on improved durability and reduced immunogenicity.
Caribou Biosciences (4-8%)
Caribou is innovating with CRISPR-based genome engineering to create next-generation, highly targeted immunotherapies.
Several biotech firms, pharmaceutical companies, and research institutions are contributing to the growth of the allogeneic T cell therapies market. These include:
The overall market size for Allogeneic T Cell Therapies Market was USD 1.4 Billion in 2025.
The Allogeneic T Cell Therapies Market expected to reach USD 3.5 Billion in 2035.
The demand for Allogeneic T Cell Therapies will be driven by the rising prevalence of haematological malignancies, advancements in gene editing technologies like CRISPR, increasing interest in off-the-shelf cell therapies, favourable regulatory approvals, and expanding biopharmaceutical R&D investments.
The top 5 countries which drives the development of Allogeneic T Cell Therapies Market are USA, UK, Europe Union, Japan and South Korea.
T Cell Receptor (TCR)-Based Therapy drives to command significant share over the assessment period.
The global allogeneic T cell therapies market has been segregated based on antigen type, indication, end-user and geography.
Protein Diagnostics Market Share, Size and Forecast 2025 to 2035
Intraoperative Fluorescence Imaging Market Report - Demand, Trends & Industry Forecast 2025 to 2035
Lung Cancer PCR Panel Market Trends, Growth, Demand & Forecast 2025 to 2035
Polymyxin Resistance Testing Market Trends – Innovations & Growth 2025 to 2035
Procalcitonin (PCT) Assay Market Analysis by Component, Type, and Region - Forecast for 2025 to 2035
Cardiovascular Diagnostics Market Report- Trends & Innovations 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.