The Alcoholic Hepatitis Treatment Market is valued at USD 3.18 Billion in 2025. As per FMI's analysis, the Alcoholic Hepatitis Treatment Industry will grow at a CAGR of 6.5% and reach USD 6.05 Billion by 2035.
In 2024, the industry experienced steady yet targeted developments across several fronts. One key driver was the rising incidence of alcohol-related liver complications, particularly in urban populations with increased alcohol consumption. On the treatment side, corticosteroids continued to be the most widely prescribed therapy, but their limitations prompted a surge in research around biologics and anti-inflammatory alternatives.
Several mid-sized pharmaceutical companies entered Phase II trials with novel compounds targeting cytokine pathways. In parallel, academic institutions partnered with biotech firms to explore gut-liver axis mechanisms, aiming to find safer long-term therapies.
Geographically, North America maintained its lead due to better diagnostic infrastructure and active government initiatives to address alcohol misuse. Meanwhile, Asia Pacific emerged as a high-growth region, with growing investments in public healthcare and rising awareness campaigns in countries like India, China, and Thailand. Hospitals and liver care centers reported a higher number of treated cases, aided by improved health insurance coverage.
Moving into 2025 and beyond, the industry is expected to be shaped by continued innovation, greater availability of targeted therapies, and a shift toward earlier diagnosis and personalized treatment models across global healthcare systems.
Market Value Insights
| Metric | Value |
|---|---|
| Industry Size (2025E) | USD 3.18 billion |
| Industry Value (2035F) | USD 6.05 billion |
| CAGR (2025 to 2035) | 6.5% |
The Alcoholic Hepatitis Treatment Industry is on a steady growth trajectory, driven by rising global alcohol consumption and increasing awareness of liver-related health risks. Advances in biologic therapies and improved diagnostic practices are reshaping treatment approaches. Pharmaceutical companies developing targeted, steroid-free solutions stand to benefit the most, while regions with weak healthcare infrastructure may lag behind.
Accelerate Biologic Drug Development
Invest in R&D for biologic and non-steroid-based therapies to address the limitations of current corticosteroid treatments and tap into emerging demand for safer, long-term solutions.
Align with Early Diagnosis Trends
Collaborate with diagnostic technology providers and public health programs to enable earlier detection and intervention, improving patient outcomes and expanding the addressable industry.
Expand Regional Footprint through Strategic Partnerships
Form partnerships with hospitals, liver care centers, and distributors in high-growth regions like Asia Pacific to strengthen industry presence and streamline last-mile treatment access.
| Risk | Probability-Impact |
|---|---|
| Regulatory delays for new biologic therapies | Medium-High |
| Limited access to advanced treatment in low-income regions | High-Medium |
| Slow adoption of early diagnostic tools by healthcare providers | Medium-Medium |
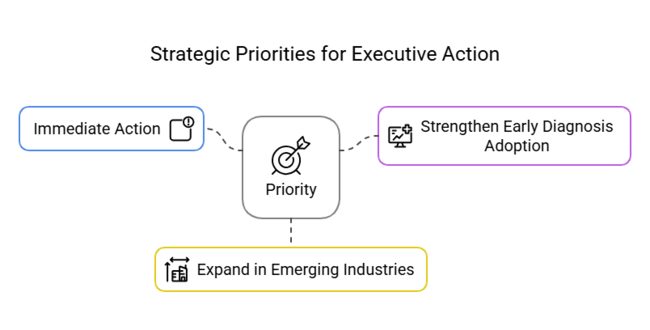
1 year Executive Watchlist
| Priority | Immediate Action |
|---|---|
| Advance Biologic Therapy Pipeline | Conduct feasibility studies and fast-track trials for non-steroid treatments |
| Strengthen Early Diagnosis Adoption | Initiate pilot programs with diagnostic firms to deploy screening tools in key hospitals |
| Expand in Emerging Industries | Launch regional partner onboarding and incentive programs in Asia Pacific |
To stay ahead, companies must accelerate investment in biologic drug development and shift focus toward early-stage intervention strategies that reflect changing clinical and regulatory dynamics. This intelligence highlights a growing industry opportunity driven by unmet patient needs, especially in regions like Asia Pacific where access is improving rapidly.
Executives should revise their roadmap to prioritize faster time-to-industry for novel therapies, build diagnostic partnerships to support early detection, and expand regional reach through local alliances. A proactive, innovation-led approach will differentiate industry leaders and position them to capture significant share as the Alcoholic Hepatitis Treatment Industry evolves over the next decade.
Surveyed Q4 2024, n=500 stakeholder participants including clinicians, hospital administrators, pharma executives, and public health policymakers across the USA, Western Europe, India, China, and Japan
Regional Variance
High Variance
ROI Perspectives
74% of USA and European executives believe tech adoption will reduce long-term costs, while 65% of stakeholders in Asia remain cautious due to budgetary constraints.
Consensus
Corticosteroids: Still the most widely used treatment globally (used by 68%), despite recognized limitations.
Variance
Shared Concerns
81% of respondents flagged high treatment costs as a barrier to access, particularly in middle-income regions.
Regional Differences
Pharma Manufacturers
Hospitals & Administrators
Public Health Policymakers
Global Alignment
73% of pharmaceutical stakeholders plan to invest in biologics and targeted cytokine therapy pipelines.
Divergence
USA
66% of stakeholders say FDA’s accelerated approval framework for liver drugs in 2024 has created optimism and fast-tracking opportunities.
Western Europe
79% credit EU's Liver Disease Prevention Strategy (2023 to 2027) for incentivizing innovation.
India/China
Only 37% reported strong regulatory influence on prescribing trends, citing inconsistent policy implementation.
High Consensus
There is widespread alignment on the need for earlier diagnosis, affordability, and the limitations of current corticosteroid therapies.
Key Variances
Strategic Insight
A region-specific approach is critical. Innovators must customize go-to-industry strategies-biologics and AI in the West, affordability and training support in Asia-to penetrate and scale in this diverse industry landscape.
| Countries | Regulatory Body and Mandatory Certifications |
|---|---|
| United States | Regulated by the FDA. Companies must comply with FDA drug approval protocols, including IND and NDA submissions. HIPAA regulations impact clinical data handling. |
| United Kingdom | Regulated by the MHRA. All medicines must be approved through the UK Market ing Authorisation system. NICE provides treatment guidelines. |
| France | ANSM regulates the industry. Mandatory CE marking for treatment equipment and strict ethical approvals for clinical trials. |
| Germany | Regulated by BfArM and the Paul Ehrlich Institute. Requires AMNOG process for reimbursement and industry access. |
| Italy | Overseen by AIFA. Requires clinical trial registration and adherence to EU-GMP guidelines for drug manufacturing. |
| South Korea | MFDS regulates drugs and therapies. Requires Pre- Industry Authorization and adherence to the Korean GMP standards. |
| Japan | MHLW and PMDA oversee regulations. Requires comprehensive safety data and post- industry surveillance as per the JPMA framework. |
| China | Regulated by the NMPA. Requires approval through Clinical Trial Application (CTA) and New Drug Application (NDA). |
| Australia-NZ | Overseen by TGA (Australia) and Medsafe (NZ). Requires ARTG listing for drugs and compliance with local clinical trial frameworks. |
Sales in the USA are anticipated to grow at a CAGR of 7.5% during 2025 to 2035.The United States remains one of the most advanced and lucrative industries for alcoholic hepatitis treatment, driven by high disease prevalence, better screening rates, and strong healthcare infrastructure. Increasing alcohol consumption patterns and rising awareness around liver-related disorders continue to push demand.
Moreover, growing research into liver regeneration therapies and clinical trials for targeted biologics positions the USA at the forefront of treatment innovation. Payers and insurers are increasingly recognizing alcoholic hepatitis as a chronic, recurring condition, which further facilitates access to treatment. The presence of major pharmaceutical companies and research institutions ensures continued R&D investments.
Sales in the UK are anticipated to grow at a CAGR of 6.8% during 2025 to 2035.In the United Kingdom, the demand for alcoholic hepatitis treatment is growing steadily, fueled by increasing incidence rates, particularly among middle-aged and younger adults. Public health campaigns and NHS initiatives focusing on alcohol misuse are improving diagnosis and early intervention. Additionally, government funding in liver disease research has been increasing, which supports the development of more effective therapies.
While the UK has a strong regulatory framework, the time-to-industry for novel biologics remains a challenge. Nonetheless, the presence of local biotech start-ups and partnerships with universities is enabling faster innovation in treatment approaches, especially around personalized medicine and inflammation-targeting drugs.
Sales in France are anticipated to grow at a CAGR of 6.8% during 2025 to 2035.France’s alcoholic hepatitis treatment industry is supported by a high per capita alcohol consumption rate, which translates into a large patient base. The country benefits from a universal healthcare system that supports widespread access to diagnostics and treatment. In recent years, French researchers have been involved in multiple EU-funded projects aimed at improving liver disease outcomes, including those related to fibrosis and cirrhosis.
While biologics adoption is still limited compared to other Western countries, generics and corticosteroids remain the primary treatment. With increased emphasis on reducing alcohol-related health burdens, demand for early intervention and effective long-term care is expected to rise.
Sales in Germany are anticipated to grow at a CAGR of 6.8% during 2025 to 2035.Germany is emerging as a prominent European hub for alcoholic hepatitis treatment, thanks to its robust hospital network and advanced pharmaceutical sector. German healthcare professionals are early adopters of emerging liver therapies, including immune-modulating drugs.
Germany’s health insurance coverage and reimbursement landscape also support wider adoption of advanced treatment options. Additionally, the country has one of the highest R&D expenditures in Europe, and several German biotech companies are developing anti-inflammatory and anti-fibrotic treatments. The prevalence of alcohol-related liver disorders, particularly among aging populations, continues to drive clinical demand, while regulatory clarity ensures a smoother path for new treatment rollouts.
Sales in Italy are anticipated to grow at a CAGR of 6.8% over the period of 2025 to 2035. The Italian alcoholic hepatitis treatment industry is gradually increasing due to the growing awareness over the long-term effects of chronic alcohol consumption. While the country has historically turned to corticosteroids and nutritional interventions, there has been a recent increase in interest in biologics and targeted anti-inflammatory medications.
The patients base is growing due to the aging population and cultural patterns related to alcohol consumption in Italy. Also, hospitals in Northern Italy are working with European research networks to test new therapies. If challenges remain, including regional differences in access to health systems and uneven diffusion of new drugs, public health initiatives, as well as EU support, will gradually address these issues.
Sales in South Korea are anticipated to grow at a CAGR of approximately 5.0% during 2025 to 2035.South Korea is experiencing moderate growth in alcoholic hepatitis treatment adoption, spurred by changing lifestyle habits and increasing alcohol consumption, especially among younger demographics. While public health awareness about liver health is rising, cultural stigma around alcohol-related diseases still limits early diagnosis.
However, South Korea’s technologically advanced healthcare system and government-backed digital health initiatives offer a favorable environment for monitoring and managing chronic liver diseases. Local biotech firms are also investing in next-generation biologics, though affordability and insurance coverage remain concerns. As public education improves and treatment guidelines evolve, South Korea is poised to see steady industry expansion over the next decade.
Sales in Japan are anticipated to grow at a CAGR of 4.5% during 2025 to 2035.Japan’s alcoholic hepatitis treatment industry is growing at a relatively slower pace, primarily due to lower disease detection rates and high reliance on traditional therapeutic approaches. Japanese physicians often favour conservative treatment methods, including dietary adjustments and basic anti-inflammatory drugs.
However, an aging population and a gradual increase in alcohol-related liver conditions are beginning to drive demand for more sophisticated interventions. Regulatory conservatism slows the introduction of foreign biologics, but domestic pharmaceutical players are active in clinical research. Continued government funding in chronic disease management and aging-related healthcare may help accelerate treatment adoption in the years ahead.
Sales in China are anticipated to grow at a CAGR of 9.2% during 2025 to 2035.China represents the most dynamic and fastest-growing alcoholic hepatitis treatment industry globally. Rapid urbanization, lifestyle changes, and rising alcohol consumption have contributed to a sharp increase in liver-related diseases. China’s government is prioritizing liver health as part of broader non-communicable disease strategies.
The country also has one of the most active pipelines for generic drugs, alongside growing interest in Western biologics and traditional Chinese medicine combinations. Expanding healthcare access in Tier 2 and Tier 3 cities is improving diagnosis rates. With strong policy support, local innovation, and increasing public health awareness, China is set to lead growth in this segment.
Sales in Australia and New Zealand are anticipated to grow at a CAGR of approximately 9.2% during 2025 to 2035.Australia and New Zealand are experiencing significant growth in the alcoholic hepatitis treatment industry, primarily due to high alcohol consumption rates and rising liver disease cases.
Public health initiatives and improved screening efforts have increased early diagnosis, while both countries have made strides in integrating advanced treatments like biologics into their national healthcare systems. Australia, in particular, has seen partnerships between hospitals and academic institutions to trial next-generation liver therapies.
New Zealand's focus on rural healthcare access is also improving reach. Both industries benefit from strong government support and progressive reimbursement models, which are accelerating the uptake of novel treatments.
Stem Cell Therapy holds the highest CAGR among all treatment segments, estimated at 8.5% for 2025 to 2035. Sales in this segment are anticipated to grow rapidly due to its regenerative capabilities, offering a potential cure rather than just symptom management.
This therapy stands out for its ability to repair liver tissue and modulate immune responses, attracting attention from both researchers and healthcare providers. With corticosteroids facing limitations in long-term effectiveness, stem cell therapy emerges as a transformative option, especially for severe cases. As more clinical trials show promising results, it is expected to become a cornerstone in the evolving landscape of alcoholic hepatitis treatment.
Injectable administration is gaining traction in alcoholic hepatitis care, showing a solid CAGR of 6.9% from 2025 to 2035. Stem cell therapies, which are typically delivered via injection, contribute heavily to this growth. The precision and effectiveness of injectable treatments are favored by clinicians for delivering targeted therapies directly to affected tissues.
Hospitals and advanced care centers are equipping themselves to handle these specialized administrations, further driving adoption. Additionally, the rise in outpatient care and improved injectable formulations have made it easier for patients to access these treatments, fueling steady demand and positioning injectables as a key administration route in the coming decade.
Stem Cell Centers are projected to grow at a strong CAGR of 8.2%, aligning closely with the surge in stem cell therapy adoption. These centers offer specialized care, infrastructure, and expertise essential for administering complex therapies like stem cell infusions.
Their role is becoming more prominent due to rising demand for advanced treatment modalities and growing trust among patients. Additionally, partnerships between biotech companies and stem cell centers are enhancing service reach and clinical trial participation. As awareness spreads and regulatory frameworks support advanced therapies, these centers will play a critical role in shaping the future of alcoholic hepatitis treatment delivery.
In 2024, companies in the alcoholic hepatitis treatment industry focused on innovation, strategic partnerships, and expanding their pipelines to gain a competitive edge. Many are working on therapies that address the underlying causes of the disease rather than just symptoms.
DURECT Corporation made headlines when its drug candidate larsucosterol received Breakthrough Therapy designation from the USA FDA, signaling faster development and regulatory review. Aldeyra Therapeutics expanded its clinical trials to include alcoholic hepatitis, strengthening its position in the liver disease segment.
Zealand Pharma and Boehringer Ingelheim also received regulatory recognition for a liver disease drug, showing how competition is heating up in related segments. Gilead, Pfizer, and Sanofi continue to maintain strong industry presence by focusing on research and partnerships. Overall, 2024 saw rising momentum in the industry, with a clear focus on newer and more effective treatments.
Market Share
F. Hoffmann-La Roche Ltd (Genentech, Inc)
Teva Pharmaceuticals
Pfizer
Merck KGaA
Sun Pharmaceutical Industries Ltd
Hikma Pharmaceuticals PLC
Viatris Inc
Alkermes, Inc
Mallinckrodt
Glenmark Pharmaceuticals USA Inc
The industry is segmented into drug treatment, stem cell therapy
The industry is divided into oral, injectable
The industry is segmented into institutional sales, retail sales
The industry is studied across North America, Latin America, Europe, East Asia, South Asia, Oceania, Middle East and Africa (MEA)
The alcoholic hepatitis treatment industry is valued at USD 3.18 billion in 2025.
The industry is projected to grow at a CAGR of 6.5% from 2025 to 2035.
North America leads the industry due to strong diagnostics and public health initiatives.
The industry is shifting toward biologic therapies and personalized treatment models.
Asia Pacific shows strong potential due to rising healthcare investments and awareness.
Table 01: Global Market Analysis 2012 to 2022 and Forecast 2023 to 2033, by Treatment
Table 02: Global Market Analysis 2012 to 2022 and Forecast 2023 to 2033by Route of Administration
Table 03: Global Market Analysis 2012 to 2022 and Forecast 2023 to 2033, by Distribution Channel
Table 04: Global Market Analysis 2012 to 2022 and Forecast 2023 to 2033, by Region
Table 05: North America Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Country
Table 06: North America Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Treatment
Table 07: North America Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Route of Administration
Table 08: North America Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Distribution Channel
Table 09: Latin America Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Country
Table 10: Latin America Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Treatment
Table 11: Latin America Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Route of Administration
Table 12: Latin America Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Distribution Channel
Table 13: Europe Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Country
Table 14: Europe Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Treatment
Table 15: Europe Market Analysis 2012 to 2022 and Forecast 2023 to 2033by Route of Administration
Table 16: Europe Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Distribution Channel
Table 17: East Asia Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Country
Table 18: East Asia Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Treatment
Table 19: East Asia Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Route of Administration
Table 20: East Asia Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Distribution Channel
Table 21: South Asia Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Country
Table 22: South Asia Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Treatment
Table 23: South Asia Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Route of Administration
Table 24: South Asia Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Distribution Channel
Table 25: Oceania Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Country
Table 26: Oceania Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Treatment
Table 27: Oceania Market Analysis 2012 to 2022 and Forecast 2023 to 2033by Route of Administration
Table 28: Oceania Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Distribution Channel
Table 29: Middle East & Africa Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Country
Table 30: Middle East & Africa Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Treatment
Table 31: Middle East & Africa Market Analysis 2012 to 2022 and Forecast 2023 to 2033by Route of Administration
Table 32: Middle East & Africa Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Distribution Channel
Figure 01: Global Market Value (US$ Million) Analysis, 2012 to 2022
Figure 02: Global Market Forecast & Y-o-Y Growth, 2023 to 2033
Figure 03: Global Market Absolute $ Opportunity (US$ Million) Analysis, 2022 to 2033
Figure 04: Global Market Value Share (%) Analysis 2023 and 2033, by Treatment
Figure 05: Global Market Y-o-Y Growth (%) Analysis 2022-2033, by Treatment
Figure 06: Global Market Attractiveness Analysis 2023 to 2033, by Treatment
Figure 07: Global Market Value Share (%) Analysis 2023 and 2033, by Route of Administration
Figure 08: Global Market Y-o-Y Growth (%) Analysis 2022-2033, by Route of Administration
Figure 09: Global Market Attractiveness Analysis 2023 to 2033, by Route of Administration
Figure 10: Global Market Value Share (%) Analysis 2023 and 2033, by Distribution Channel
Figure 11: Global Market Y-o-Y Growth (%) Analysis 2022-2033, by Distribution Channel
Figure 12: Global Market Attractiveness Analysis 2023 to 2033, by Distribution Channel
Figure 13: Global Market Value Share (%) Analysis 2023 and 2033, by Region
Figure 14: Global Market Y-o-Y Growth (%) Analysis 2022-2033, by Region
Figure 15: Global Market Attractiveness Analysis 2023 to 2033, by Region
Figure 16: North America Market Value (US$ Million) Analysis, 2012 to 2022
Figure 17: North America Market Value (US$ Million) Forecast, 2023-2033
Figure 18: North America Market Value Share, by Treatment (2023 E)
Figure 19: North America Market Value Share, by Route of Administration (2023 E)
Figure 20: North America Market Value Share, by Distribution Channel (2023 E)
Figure 21: North America Market Value Share, by Country (2023 E)
Figure 22: North America Market Attractiveness Analysis by Treatment, 2023 to 2033
Figure 23: North America Market Attractiveness Analysis by Route of Administration, 2023 to 2033
Figure 24: North America Market Attractiveness Analysis by Distribution Channel, 2023 to 2033
Figure 25: North America Market Attractiveness Analysis by Country, 2023 to 2033
Figure 26: USA Market Value Proportion Analysis, 2022
Figure 27: Global Vs. USA Growth Comparison, 2022 to 2033
Figure 28: USA Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 29: USA Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 30: USA Market Share Analysis (%) by Distribution Channel, 2022 & 2033
Figure 31: Canada Market Value Proportion Analysis, 2022
Figure 32: Global Vs. Canada. Growth Comparison, 2022 to 2033
Figure 33: Canada Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 34: Canada Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 35: Canada Market Share Analysis (%) by Distribution Channel, 2022 & 2033
Figure 36: Latin America Market Value (US$ Million) Analysis, 2012 to 2022
Figure 37: Latin America Market Value (US$ Million) Forecast, 2023-2033
Figure 38: Latin America Market Value Share, by Treatment (2023 E)
Figure 39: Latin America Market Value Share, by Route of Administration (2023 E)
Figure 40: Latin America Market Value Share, by Distribution Channel (2023 E)
Figure 41: Latin America Market Value Share, by Country (2023 E)
Figure 42: Latin America Market Attractiveness Analysis by Treatment, 2023 to 2033
Figure 43: Latin America Market Attractiveness Analysis by Route of Administration, 2023 to 2033
Figure 44: Latin America Market Attractiveness Analysis by Distribution Channel, 2023 to 2033
Figure 45: Latin America Market Attractiveness Analysis by Country, 2023 to 2033
Figure 46: Mexico Market Value Proportion Analysis, 2022
Figure 47: Global Vs Mexico Growth Comparison, 2022 to 2033
Figure 48: Mexico Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 49: Mexico Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 50: Mexico Market Share Analysis (%) by Distribution Channel, 2022 & 2033
Figure 51: Brazil Market Value Proportion Analysis, 2022
Figure 52: Global Vs. Brazil. Growth Comparison, 2022 to 2033
Figure 53: Brazil Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 54: Brazil Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 55: Brazil Market Share Analysis (%) by Distribution Channel, 2022 & 2033
Figure 56: Argentina Market Value Proportion Analysis, 2022
Figure 57: Global Vs Argentina Growth Comparison, 2022 to 2033
Figure 58: Argentina Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 59: Argentina Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 60: Argentina Market Share Analysis (%) by Distribution Channel, 2022 & 2033
Figure 61: Europe Market Value (US$ Million) Analysis, 2012 to 2022
Figure 62: Europe Market Value (US$ Million) Forecast, 2023-2033
Figure 63: Europe Market Value Share, by Treatment (2023 E)
Figure 64: Europe Market Value Share, by Route of Administration (2023 E)
Figure 65: Europe Market Value Share, by Distribution Channel (2023 E)
Figure 66: Europe Market Value Share, by Country (2023 E)
Figure 67: Europe Market Attractiveness Analysis by Treatment, 2023 to 2033
Figure 68: Europe Market Attractiveness Analysis by Route of Administration, 2023 to 2033
Figure 69: Europe Market Attractiveness Analysis by Distribution Channel, 2023 to 2033
Figure 70: Europe Market Attractiveness Analysis by Country, 2023 to 2033
Figure 71: UK Market Value Proportion Analysis, 2022
Figure 72: Global Vs. UK Growth Comparison, 2022 to 2033
Figure 73: UK Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 74: UK Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 75: UK Market Share Analysis (%) by Distribution Channel, 2022 & 2033
Figure 76: Germany Market Value Proportion Analysis, 2022
Figure 77: Global Vs. Germany Growth Comparison, 2022 to 2033
Figure 78: Germany Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 79: Germany Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 80: Germany Market Share Analysis (%) by Distribution Channel, 2022 & 2033
Figure 81: Italy Market Value Proportion Analysis, 2022
Figure 82: Global Vs. Italy Growth Comparison, 2022 to 2033
Figure 83: Italy Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 84: Italy Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 85: Italy Market Share Analysis (%) by Distribution Channel, 2022 & 2033
Figure 86: France Market Value Proportion Analysis, 2022
Figure 87: Global Vs France Growth Comparison, 2022 to 2033
Figure 88: France Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 89: France Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 90: France Market Share Analysis (%) by Distribution Channel, 2022 & 2033
Figure 91: Spain Market Value Proportion Analysis, 2022
Figure 92: Global Vs Spain Growth Comparison, 2022 to 2033
Figure 93: Spain Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 94: Spain Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 95: Spain Market Share Analysis (%) by Distribution Channel, 2022 & 2033
Figure 96: Russia Market Value Proportion Analysis, 2022
Figure 97: Global Vs Russia Growth Comparison, 2022 to 2033
Figure 98: Russia Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 99: Russia Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 100: Russia Market Share Analysis (%) by Distribution Channel, 2022 & 2033
Figure 101: BENELUX Market Value Proportion Analysis, 2022
Figure 102: Global Vs BENELUX Growth Comparison, 2022 to 2033
Figure 103: BENELUX Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 104: BENELUX Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 105: BENELUX Market Share Analysis (%) by Distribution Channel, 2022 & 2033
Figure 106: East Asia Market Value (US$ Million) Analysis, 2012 to 2022
Figure 107: East Asia Market Value (US$ Million) Forecast, 2023-2033
Figure 108: East Asia Market Value Share, by Treatment (2023 E)
Figure 109: East Asia Market Value Share, by Route of Administration (2023 E)
Figure 110: East Asia Market Value Share, by Distribution Channel (2023 E)
Figure 111: East Asia Market Value Share, by Country (2023 E)
Figure 112: East Asia Market Attractiveness Analysis by Treatment, 2023 to 2033
Figure 113: East Asia Market Attractiveness Analysis by Route of Administration, 2023 to 2033
Figure 114: East Asia Market Attractiveness Analysis by Distribution Channel, 2023 to 2033
Figure 115: East Asia Market Attractiveness Analysis by Country, 2023 to 2033
Figure 116: China Market Value Proportion Analysis, 2022
Figure 117: Global Vs. China Growth Comparison, 2022 to 2033
Figure 118: China Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 119: China Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 120: China Market Share Analysis (%) by Distribution Channel, 2022 & 2033
Figure 121: Japan Market Value Proportion Analysis, 2022
Figure 122: Global Vs. Japan Growth Comparison, 2022 to 2033
Figure 123: Japan Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 124: Japan Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 125: Japan Market Share Analysis (%) by Distribution Channel, 2022 & 2033
Figure 126: South Korea Market Value Proportion Analysis, 2022
Figure 127: Global Vs South Korea Growth Comparison, 2022 to 2033
Figure 128: South Korea Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 129: South Korea Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 130: South Korea Market Share Analysis (%) by Distribution Channel, 2022 & 2033
Figure 131: South Asia Market Value (US$ Million) Analysis, 2012 to 2022
Figure 132: South Asia Market Value (US$ Million) Forecast, 2023-2033
Figure 133: South Asia Market Value Share, by Treatment (2023 E)
Figure 134: South Asia Market Value Share, by Route of Administration (2023 E)
Figure 135: South Asia Market Value Share, by Distribution Channel (2023 E)
Figure 136: South Asia Market Value Share, by Country (2023 E)
Figure 137: South Asia Market Attractiveness Analysis by Treatment, 2023 to 2033
Figure 138: South Asia Market Attractiveness Analysis by Route of Administration, 2023 to 2033
Figure 139: South Asia Market Attractiveness Analysis by Distribution Channel, 2023 to 2033
Figure 140: South Asia Market Attractiveness Analysis by Country, 2023 to 2033
Figure 141: India Market Value Proportion Analysis, 2022
Figure 142: Global Vs. India Growth Comparison, 2022 to 2033
Figure 143: India Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 144: India Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 145: India Market Share Analysis (%) by Distribution Channel, 2022 & 2033
Figure 146: Indonesia Market Value Proportion Analysis, 2022
Figure 147: Global Vs. Indonesia Growth Comparison, 2022 to 2033
Figure 148: Indonesia Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 149: Indonesia Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 150: Indonesia Market Share Analysis (%) by Distribution Channel, 2022 & 2033
Figure 151: Malaysia Market Value Proportion Analysis, 2022
Figure 152: Global Vs. Malaysia Growth Comparison, 2022 to 2033
Figure 153: Malaysia Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 154: Malaysia Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 155: Malaysia Market Share Analysis (%) by Distribution Channel, 2022 & 2033
Figure 156: Thailand Market Value Proportion Analysis, 2022
Figure 157: Global Vs. Thailand Growth Comparison, 2022 to 2033
Figure 158: Thailand Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 159: Thailand Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 160: Thailand Market Share Analysis (%) by Distribution Channel, 2022 & 2033
Figure 161: Oceania Market Value (US$ Million) Analysis, 2012 to 2022
Figure 162: Oceania Market Value (US$ Million) Forecast, 2023-2033
Figure 163: Oceania Market Value Share, by Treatment (2023 E)
Figure 164: Oceania Market Value Share, by Route of Administration (2023 E)
Figure 165: Oceania Market Value Share, by Distribution Channel (2023 E)
Figure 166: Oceania Market Value Share, by Country (2023 E)
Figure 167: Oceania Market Attractiveness Analysis by Treatment, 2023 to 2033
Figure 168: Oceania Market Attractiveness Analysis by Route of Administration, 2023 to 2033
Figure 169: Oceania Market Attractiveness Analysis by Distribution Channel, 2023 to 2033
Figure 170: Oceania Market Attractiveness Analysis by Country, 2023 to 2033
Figure 171: Australia Market Value Proportion Analysis, 2022
Figure 172: Global Vs. Australia Growth Comparison, 2022 to 2033
Figure 173: Australia Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 174: Australia Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 175: Australia Market Share Analysis (%) by Distribution Channel, 2022 & 2033
Figure 176: New Zealand Market Value Proportion Analysis, 2022
Figure 177: Global Vs New Zealand Growth Comparison, 2022 to 2033
Figure 178: New Zealand Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 179: New Zealand Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 180: New Zealand Market Share Analysis (%) by Distribution Channel, 2022 & 2033
Figure 181: Middle East & Africa Market Value (US$ Million) Analysis, 2012 to 2022
Figure 182: Middle East & Africa Market Value (US$ Million) Forecast, 2023-2033
Figure 183: Middle East & Africa Market Value Share, by Treatment (2023 E)
Figure 184: Middle East & Africa Market Value Share, by Route of Administration (2023 E)
Figure 185: Middle East & Africa Market Value Share, by Distribution Channel (2023 E)
Figure 186: Middle East & Africa Market Value Share, by Country (2023 E)
Figure 187: Middle East & Africa Market Attractiveness Analysis by Treatment, 2023 to 2033
Figure 188: Middle East & Africa Market Attractiveness Analysis by Route of Administration, 2023 to 2033
Figure 189: Middle East & Africa Market Attractiveness Analysis by Distribution Channel, 2023 to 2033
Figure 190: Middle East & Africa Market Attractiveness Analysis by Country, 2023 to 2033
Figure 191: GCC Countries Market Value Proportion Analysis, 2022
Figure 192: Global Vs GCC Countries Growth Comparison, 2022 to 2033
Figure 193: GCC Countries Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 194: GCC Countries Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 195: GCC Countries Market Share Analysis (%) by Distribution Channel, 2022 & 2033
Figure 196: Türkiye Market Value Proportion Analysis, 2022
Figure 197: Global Vs. Türkiye Growth Comparison, 2022 to 2033
Figure 198: Türkiye Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 199: Türkiye Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 200: Türkiye Market Share Analysis (%) by Distribution Channel, 2022 & 2033
Figure 201: South Africa Market Value Proportion Analysis, 2022
Figure 202: Global Vs. South Africa Growth Comparison, 2022 to 2033
Figure 203: South Africa Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 204: South Africa Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 205: South Africa Market Share Analysis (%) by Distribution Channel, 2022 & 2033
Figure 206: North Africa Market Value Proportion Analysis, 2022
Figure 207: Global Vs North Africa Growth Comparison, 2022 to 2033
Figure 208: North Africa Market Share Analysis (%) by Treatment, 2022 & 2033
Figure 209: North Africa Market Share Analysis (%) by Route of Administration, 2022 & 2033
Figure 210: North Africa Market Share Analysis (%) by Distribution Channel, 2022 & 2033






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Alcoholic Drinks Packaging Market Size and Share Forecast Outlook 2025 to 2035
Alcoholic Flavors Market Size, Growth, and Forecast for 2025 to 2035
Alcoholic Ice Cream Market
Non Alcoholic RTD Beverages Market Size and Share Forecast Outlook 2025 to 2035
Non-Alcoholic Beer Market Insights - Trends, Demand & Growth 2025 to 2035
Non-Alcoholic Malt Beverages Market Size, Growth, and Forecast for 2025 to 2035
Industry Share & Competitive Positioning in Non-Alcoholic Malt Beverages
Non-Alcoholic Steatohepatitis Clinical Trials Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Non-alcoholic Steatohepatitis Drugs Pipeline Market Outlook 2025 to 2035
UK Non-Alcoholic Malt Beverages Market Analysis from 2025 to 2035
Canned Alcoholic Beverages Market Analysis by Product Type, Distribution Channel, and Region Through 2035
Premium Alcoholic Beverage Market - Size, Share, and Forecast 2025 to 2035
USA Non-Alcoholic Malt Beverages Market Insights – Trends, Demand & Growth 2025-2035
ASEAN Non-Alcoholic Malt Beverages Market Trends – Demand & Forecast 2025–2035
Europe Non-Alcoholic Malt Beverages Market Insights – Demand & Growth 2025–2035
Pre-mixed/RTD Alcoholic Drink Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Australia Non-Alcoholic Malt Beverages Market Insights - Trends & Forecast 2025 to 2035
Latin America Non-Alcoholic Malt Beverages Market Trends – Growth & Forecast 2025–2035
Hepatitis C Virus (HCV) Testing Market Size and Share Forecast Outlook 2025 to 2035
Hepatitis - B Therapeutics Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA