The adrenal crisis management industry is valued at USD 3.76 million in 2025. As per FMI's analysis, the industry will grow at a CAGR of 6.7% and reach USD 7.19 million by 2035.
In 2024, the adrenal crisis also known as acute adrenal insufficiency management industry saw significant advancements in how people were treated. This was due to more doctors learning about the problem and easier access to emergency drugs.
The research also indicates that regulatory approval timelines for new hydrocortisone products and prefilled syringes, especially in North America and Europe, have shortened. As people sought better healthcare solutions, demand for wearable medical alert devices increased. Delayed diagnoses continue to contribute to high hospitalization rates, highlighting the need for greater investment in early detection programs.

Moving forward to 2025, business growth will speed up as telemedicine platforms adopt adrenal crisis management software to improve care for patients. Drug companies will spend money on AI-assisted drug formulation to make corticosteroids work better.
In the future, beyond 2025, Asian, Pacific, and Latin American markets will become increasingly influential with enhanced healthcare infrastructure and growing insurance penetration. Advancements in gene therapy and precision medicine will revolutionize acute adrenal insufficiency treatments by 2035.
The adrenal crisis management industry is expected to grow steadily over the next few years thanks to more people knowing about it, better emergency care, and easier access to drugs that can save lives. According to research by FMI, the growing need for quick solutions will be good for pharmaceutical companies and telemedicine service providers. However, places with poor healthcare infrastructure might fall behind. Improvements in corticosteroid formulations and digital health technologies will help the industry grow, and by 2035, patient outcomes will be better.
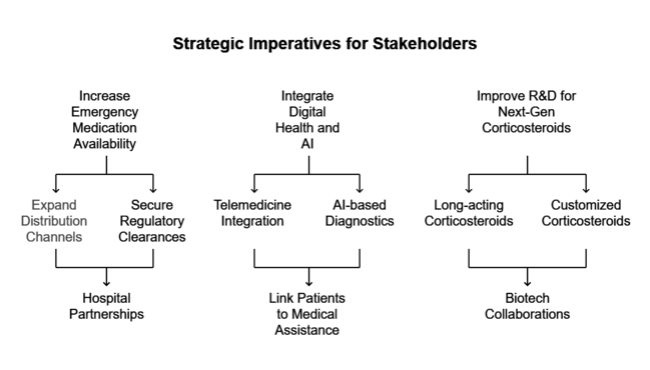
Increase Emergency Medication Availability
Executives should invest in expanding distribution channels and regulatory clearances so that areas that don't have enough access to life-saving acute adrenal insufficiency medicines can get them. Hospital and pharmacy partnerships will be essential to broadening access.
Tap digital health and AI integration
Integrating telemedicine with AI-based diagnostics will enable faster detection and treatment of adrenal crises. Companies must create digital platforms linking patients with immediate medical assistance.
Bolster R&D for Next-Gen Corticosteroids
Research and development of next-generation long-acting and customized corticosteroid drugs will fuel competitive differentiation. Biotech companies can help companies come up with new ideas and get into new scenarios faster through strategic mergers and acquisitions and co-development partnerships.
| Risk | Probability & Impact |
|---|---|
| Supply Chain Disruptions | High Probability-High Impact |
| Regulatory Hurdles | Medium Probability-High Impact |
| Patient Non-Adherence | High Probability-Medium Impact |
1-Year Executive Watchlist
| Priority | Immediate Action |
|---|---|
| Expand Access to Emergency Medications | Accelerate partnerships with hospitals and pharmacies to improve distribution |
| Enhance Digital Health Integration | Develop AI-driven telemedicine tools for real-time a cute adrenal insufficiency management |
| Strengthen Regulatory Compliance | Streamline approvals for novel corticosteroid formulations in key markets |
Executives need to make sure that everyone in the world has access to emergency treatments for adrenal crises through digital health innovation. This will improve early intervention and save lives. Strategic investments in AI-assisted diagnostics and next-generation corticosteroids will be essential to stay ahead in the long term. Obtaining regulatory clearances in high-growth scenarios and forming telemedicine alliances should thus be top priorities for gaining a competitive advantage in a changing healthcare environment.
Regional Difference
High Variance
ROI - Perspectives
69% of North American stakeholders said that “AI-driven treatment approaches” are “worth the investment,” compared to 41% in Asia-Pacific, where potential affordability issues deterred stakeholders.
Consensus
Hydrocortisone in prefilled syringes:71% preferred prefilled hydrocortisone syringes for emergency use to enhance patient accessibility.
Regional Variance
Common Concerns
87% highlighted increased production costs for drugs, including 20% increases in API prices over the past year.
Regional Differences
Manufacturers
Hospitals & Pharmacies
Alignment
76% of global pharma companies plan to invest in next-generation corticosteroids with longer half-lives.
Regional Emphasis
High Consensus
Emergency response, cost-effectiveness, and supply chain flexibility continue to be top global priorities.
Variances
Strategic Insight: A global approach is crucial-North America will emphasize high-tech solutions, Europe will focus on green practices, and Asia-Pacific will prioritize cost-effective alternatives.
| Countries | Regulatory Impact & Mandatory Certifications |
|---|---|
| United States | Hydrocortisone drug products are necessarily required under the Orphan Drug Act for the treatment of adrenal crises, with very few exceptions; however, in 2023, the EUA will fast-track approval for hydrocortisone drug products. |
| United Kingdom | Fitbit meets the requirements of the MHRA (January 22, 2023). MHRA follows the rules for pharmacovigilance set by the EU and now has its own fast-track process for life-saving medicines since the UK left the EU. |
| France | Corticosteroids must be carefully packed and labeled according to strict rules set by ANSM; they must also follow EU Good Manufacturing Practices (GMP). |
| Germany | Clinical trials are necessary for new corticosteroid formulations, and the EU Green Deal now mandates sustainability compliance. |
| Italy | AIFA's decision to put price caps on important medicines affects expensive treatments for adrenal crisis; EU pharmacovigilance guidelines must be followed. |
| South Korea | Emergency drugs, on the other hand, have to go through new clinical trials in South Korea as part of the MFDS. These trials take time because of strict safety checks. |
| Japan | In Japan, PMDA has hindered approval timelines, yet it provides fast-track designations for a cute adrenal insufficiency drugs within the Sakigake system. |
| China | China's National Medical Products Administration (NMPA) regulations favor generic hydrocortisone and require foreign drugmakers to collaborate with local partners for industry approval. |
| India | The Drug Price Control Order (DPCO) of India controls the prices of high-cost formulations and is in charge of approving corticosteroids. Cost containment is required by this order. |
The diagnosis segment of the adrenal crisis management industry is anticipated to growat a CAGR of 6.9% during the period between 2025 and 2035, headed by blood tests and ACTH stimulation tests because of their reliability and extensive clinical use. Blood tests remain the primary choice for diagnosis, resulting in consistent demand.
The ACTH stimulation test is also on the rise, as it is very reliable when testing for adrenal insufficiency. Insulin-induced hypoglycemia testing is reserved for difficult cases but is tightly regulated due to safety concerns. Imaging tests, like MRI and CT scans, are growing slowly because they help find problems with the adrenal glands but are too expensive to be used very often.
The industry is growing because of more doctors becoming aware of the problem and a greater focus on finding it early. The use of AI-based diagnostic equipment and point-of-care testing is anticipated to increase accessibility, thereby driving industry growth in this segment further.
The treatment segment for adrenal crisis is anticipated to grow at a CAGR of 7.3% during the period 2025 to 2035, with hydrocortisone dominating the industry because of its effectiveness and availability. A lot of people are still taking oral cortisone and fludrocortisone acetate, mostly to treat adrenal insufficiency over the long term.
As more people go to the hospital, intravenous injections, especially emergency hydrocortisone and corticosteroid treatments, are growing very quickly. The growing use of corticosteroids for immediate crisis intervention also fuels demand. It is important to use saline solution and sugar (dextrose) treatments for emergency situations, but their growth is limited by new developments in other drugs.
More money is being put into long-term corticosteroid products, which makes it easier for patients to stick with their treatments and improves therapeutic outcomes. More telehealth prescriptions mean that more people around the world can get access to important drugs for acute adrenal insufficiency.
The part of acute adrenal insufficiency management that deals with distribution channels is expected to grow at a CAGR of 7.1% from 2025 to 2035. Hospital pharmacies will be the leaders in this area because they help with emergency care. Hospitals are still the point of care of choice for acute adrenal insufficiency patients, necessitating the strongest demand for life-sustaining drugs.
Retail pharmacies enjoy a fixed industry share with ongoing prescription refills and convenience. However, internet pharmacies are the fastest-growing industry. This is because more people are using digital healthcare, it's easier to get cheap drugs, and the government backs e-pharmacies. This industry is growing due to the convenience of home delivery and increased insurance coverage for online purchases.
To take advantage of how the digital world is changing, companies in the pharmaceutical industry are investing more in e-commerce partnerships and direct-to-consumer platforms. As more people use telemedicine, online pharmacies are likely to become major providers of acute adrenal insufficiency management drugs.
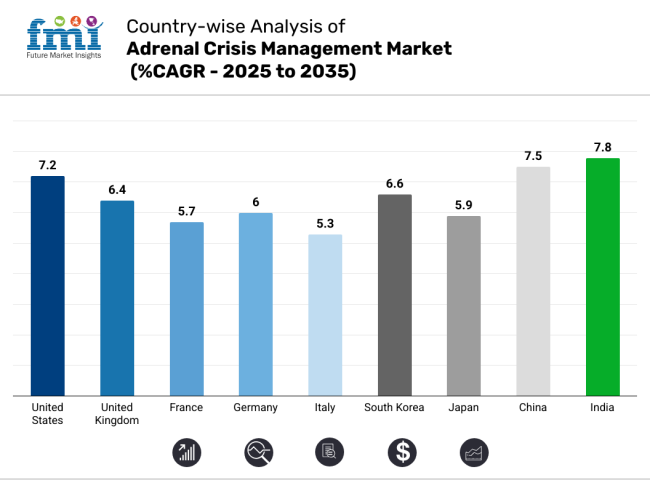
The adrenal crisis management industry in the USA is expected to grow at a compound annual growth rate (CAGR) of 7.2% between 2025 and 2035. This is because more people are learning about it, more people are using telemedicine, and the government supports life-saving emergency treatments. The FDA's fast-track approval pathways, such as the Orphan Drug Act and Emergency Use Authorization (EUA), can help these hydrocortisone formulations get to industry faster.
Advancements in technology such as AI-algorithm-assisted risk detection and smart wearable medical alert devices are revolutionizing patient management. Supply chain risks and high-priced treatments pose significant challenges. The fact that big drug companies are working on next-generation corticosteroids makes the USA much more competitive in this area.
A strong government, more freedom for regulators after Brexit, and more digital healthcare will help the acute adrenal insufficiency management industry grow at a compound annual growth rate (CAGR) of 6.4% in the UK from 2025 to 2030. The UK's Medicines and Healthcare Products Regulatory Agency (MHRA) has a rapid regulatory approach. This has accelerated the approval process for important medicines, lowering the time it takes for emergency-use corticosteroids to reach the industry.
Public health information campaigns are helpful in the earlier diagnosis and better management of adrenal insufficiency. However, price controls in the National Health Service (NHS) bring commercial value down in higher-end formulations. Sustainability efforts and green manufacturing methods for pharmaceutical packaging are becoming more and more important for companies in this industry.
The other companies in France make the industry very competitive, which helps bring in money for adrenal crisis management. From 2025 to 2035, the industry is expected to grow at a rate of 5.7% per year. This graph shows how the country's rules work and emphasizes how inexpensive it is to do things that can save lives. The ANSM has strict pharmacovigilance rules that make it difficult for new companies to play in the landscape. In a universal health system, many people want corticosteroids because they can get them easily.
Furthermore, companies are more likely to use these environmentally friendly ways of making drugs because they make the drugs last longer and are in line with EU sustainability rules that require sustainable pharmaceutical manufacturing. France's centralized system for buying medicines does raise prices, but the country's growth potential is high thanks to its heavy investment in biotech innovation.
Germany's acute adrenal insufficiency management industry is also anticipated to garner USD 448.4 million by the end of 2035, growing at a CAGR of 6.0% during the forecast period, which can be attributed to high healthcare expenditure, an advanced regulatory landscape, and a strong trend toward innovative corticosteroid products. The strict clinical validation criteria used by the BfArM make sure that products are safe and effective, which gives people confidence.
In fact, the country is at the forefront of telemedicine in Europe, improving acute adrenal insufficiency monitoring. But the costs of following the rules set by regulators and the EU's requirements for sustainability make it hard for pharmaceutical companies to run their businesses. Germany's emphasis on innovation and regulatory leadership is expected to shape the industry’s trajectory over the next decade.
From 2025 to 2035, the Italian adrenal crisis management industry is expected to grow at a compound annual growth rate (CAGR) of 5.3%. The healthcare system in Italy is strong, and the government is working to make it easier for people to get medicines. As part of the EU, AIFA makes South Korea's pharmacovigilance pricing systems stronger so that high-cost corticosteroids don't cost too much.
Italian research expenditure echoes this excitement for innovative drug delivery technology. Health efforts are making it easier to diagnose problems early, and the growth of telehealth services is making it easier to manage patients. But bureaucratic hurdles cannot negate Italy’s attempts to expand access to treatment.
South Korea’s adrenal crisis management industry is projected to grow at a CAGR of 6.6% from 2025 to 2035, driven by digital health adoption and increased R&D investment. More people are using digital health, spending more on healthcare, and the government is funding research and development. All new corticosteroids must pass strict safety tests required by the MFDS, which makes the approval process take longer.
But more and more diagnostics and telemedicine services that use AI are changing the way adrenal crises are treated. Local drug companies are coming up with new hydrocortisone formulations because the country wants to improve pharmaceuticals and offers research grants and tax breaks. South Korea's advanced healthcare infrastructure makes the country a key player in the industry, but its current pricing pressures are due to high domestic costs.
Japan's management of adrenal crises will grow at a compound annual growth rate (CAGR) of 5.9% between 2025 and 2035. This increase is because the population will change, the government will spend more, and patients will become more aware of their condition. Drug approval procedures have been in place for a long time at the PMDA. However, the Sakigake fast-track scheme speeds up the release of approved medicines that save lives.
More emergency corticosteroids are needed with the increasing geriatric population in the country. This implies a higher usage of digital health for crisis management. However, cost-sensitive healthcare policies and government price negotiations limit high-margin potential. Developments are likely to rely on the nation's push for precision medicine and biotech.
The management of adrenal crises in China will grow at a CAGR of 7.5% from 2025 to 2035. This is due to the government's significant investments in healthcare, increased awareness of the disease, and increased access to treatment. Working with local businesses and entering markets under the NMPA both make it easier and harder for drugs to be brought into the country. The national bulk procurement system in China favors cheap hydrocortisone preparations over pricey medicines, according to Zhan.
This makes it harder for expensive medicines to sell. However, the availability of acute adrenal insufficiency care is increased due to the fact that a greater number of individuals reside in urban areas and have access to more comprehensive insurance coverage. The government's plans to implement AI-powered health care solutions and to manufacture medicines in the United States will significantly impact the growth of this industry.
With a growth rate of 7.8% per year from 2025 to 2035, the Indian industry for adrenal crisis management is one of the mature markets that is growing the fastest. This boost is because more people can get medical care, more people are learning about the disease, and the government is supporting affordable solutions. The Drug Price Control Order (DPCO) makes sure that corticosteroids are affordable by setting their prices.
The CDSCO, on the other hand, is in charge of approving drugs. As an international supplier of cheap hydrocortisone, India's rapidly expanding generic drug industry is a key driver of cost-effective acute adrenal insufficiency management solutions worldwide. But the lack of healthcare representation in rural areas is a limitation. Investment in digital health and telemedicine will automate care and improve access and healing for patients.
The industry for treating adrenal crises is competitive, and the big players are changing how they do business and teaming up with others to get a bigger share of the international acute adrenal insufficiency treatment market. Key players in the industry are also very interested in economic corticosteroid products and investing in research and production of drugs that work slowly over time and are bioavailable.
Innovation (particularly in AI-based diagnostics) and integration with telemedicine are differentiators. Partnerships with hospitals, pharmacies, and digital health service providers reinforce distribution networks. Geographic expansion in high-growth regions like Asia-Pacific is a major strategy. There are also plans for mergers and acquisitions to improve product lines and stay ahead of changing laws and technologies.
Industry Share Analysis
Pfizer Inc.
Novartis AG
Ampha star Pharmaceuticals, Inc.
Hikma Pharmaceuticals PLC
Mylan N.V. (now Viatris Inc.)
Key Developments in 2024
The industry is segmented into blood test, ACTH stimulation test, insulin-induced hypoglycaemia test, imaging test
It is segmented into oral cortisone (hydrocortisone & fludrocortisone acetate) and intravenous injections (corticosteroids, saline solution & sugar (dextrose))
It is fragmented into hospital pharmacy, retail pharmacy and online pharmacy
The industry is studied across North America, Latin America, Europe, South Asia, East Asia, Oceania, Middle East & Africa.
The industry is expected to be driven by the growing incidence of adrenal insufficiency, increased awareness of acute adrenal insufficiency symptoms, and advancements in diagnostic technology.
Early diagnosis, easier access to corticosteroids that can save lives, and more people using digital healthcare solutions are all positive signs for the industry's steady growth.
Some of the leading companies are Pfizer Inc., Novartis AG, Takeda Pharmaceutical Company Limited, Sanofi S.A., and AbbVie Inc.
Hydrocortisone is still the treatment of choice, but intravenous corticosteroids are becoming more popular as they have been shown to be effective in emergency medicine.
It is expected that the industry will grow at a compound annual growth rate (CAGR) of 6.7% and reach USD 7.19 million by 2035. This is because more people are seeking to diagnose and treat adrenal disorders.
Table 1: Global Market Value (US$ Million) Forecast by Region, 2018 to 2033
Table 2: Global Market Value (US$ Million) Forecast by Diagnosis Method, 2018 to 2033
Table 3: Global Market Value (US$ Million) Forecast by Treatment Method, 2018 to 2033
Table 4: Global Market Value (US$ Million) Forecast by Distribution Channel, 2018 to 2033
Table 5: North America Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 6: North America Market Value (US$ Million) Forecast by Diagnosis Method, 2018 to 2033
Table 7: North America Market Value (US$ Million) Forecast by Treatment Method, 2018 to 2033
Table 8: North America Market Value (US$ Million) Forecast by Distribution Channel, 2018 to 2033
Table 9: Latin America Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 10: Latin America Market Value (US$ Million) Forecast by Diagnosis Method, 2018 to 2033
Table 11: Latin America Market Value (US$ Million) Forecast by Treatment Method, 2018 to 2033
Table 12: Latin America Market Value (US$ Million) Forecast by Distribution Channel, 2018 to 2033
Table 13: Europe Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 14: Europe Market Value (US$ Million) Forecast by Diagnosis Method, 2018 to 2033
Table 15: Europe Market Value (US$ Million) Forecast by Treatment Method, 2018 to 2033
Table 16: Europe Market Value (US$ Million) Forecast by Distribution Channel, 2018 to 2033
Table 17: South Asia Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 18: South Asia Market Value (US$ Million) Forecast by Diagnosis Method, 2018 to 2033
Table 19: South Asia Market Value (US$ Million) Forecast by Treatment Method, 2018 to 2033
Table 20: South Asia Market Value (US$ Million) Forecast by Distribution Channel, 2018 to 2033
Table 21: East Asia Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 22: East Asia Market Value (US$ Million) Forecast by Diagnosis Method, 2018 to 2033
Table 23: East Asia Market Value (US$ Million) Forecast by Treatment Method, 2018 to 2033
Table 24: East Asia Market Value (US$ Million) Forecast by Distribution Channel, 2018 to 2033
Table 25: Oceania Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 26: Oceania Market Value (US$ Million) Forecast by Diagnosis Method, 2018 to 2033
Table 27: Oceania Market Value (US$ Million) Forecast by Treatment Method, 2018 to 2033
Table 28: Oceania Market Value (US$ Million) Forecast by Distribution Channel, 2018 to 2033
Table 29: MEA Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 30: MEA Market Value (US$ Million) Forecast by Diagnosis Method, 2018 to 2033
Table 31: MEA Market Value (US$ Million) Forecast by Treatment Method, 2018 to 2033
Table 32: MEA Market Value (US$ Million) Forecast by Distribution Channel, 2018 to 2033
Figure 1: Global Market Value (US$ Million) by Diagnosis Method, 2023 to 2033
Figure 2: Global Market Value (US$ Million) by Treatment Method, 2023 to 2033
Figure 3: Global Market Value (US$ Million) by Distribution Channel, 2023 to 2033
Figure 4: Global Market Value (US$ Million) by Region, 2023 to 2033
Figure 5: Global Market Value (US$ Million) Analysis by Region, 2018 to 2033
Figure 6: Global Market Value Share (%) and BPS Analysis by Region, 2023 to 2033
Figure 7: Global Market Y-o-Y Growth (%) Projections by Region, 2023 to 2033
Figure 8: Global Market Value (US$ Million) Analysis by Diagnosis Method, 2018 to 2033
Figure 9: Global Market Value Share (%) and BPS Analysis by Diagnosis Method, 2023 to 2033
Figure 10: Global Market Y-o-Y Growth (%) Projections by Diagnosis Method, 2023 to 2033
Figure 11: Global Market Value (US$ Million) Analysis by Treatment Method, 2018 to 2033
Figure 12: Global Market Value Share (%) and BPS Analysis by Treatment Method, 2023 to 2033
Figure 13: Global Market Y-o-Y Growth (%) Projections by Treatment Method, 2023 to 2033
Figure 14: Global Market Value (US$ Million) Analysis by Distribution Channel, 2018 to 2033
Figure 15: Global Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 16: Global Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 17: Global Market Attractiveness by Diagnosis Method, 2023 to 2033
Figure 18: Global Market Attractiveness by Treatment Method, 2023 to 2033
Figure 19: Global Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 20: Global Market Attractiveness by Region, 2023 to 2033
Figure 21: North America Market Value (US$ Million) by Diagnosis Method, 2023 to 2033
Figure 22: North America Market Value (US$ Million) by Treatment Method, 2023 to 2033
Figure 23: North America Market Value (US$ Million) by Distribution Channel, 2023 to 2033
Figure 24: North America Market Value (US$ Million) by Country, 2023 to 2033
Figure 25: North America Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 26: North America Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 27: North America Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 28: North America Market Value (US$ Million) Analysis by Diagnosis Method, 2018 to 2033
Figure 29: North America Market Value Share (%) and BPS Analysis by Diagnosis Method, 2023 to 2033
Figure 30: North America Market Y-o-Y Growth (%) Projections by Diagnosis Method, 2023 to 2033
Figure 31: North America Market Value (US$ Million) Analysis by Treatment Method, 2018 to 2033
Figure 32: North America Market Value Share (%) and BPS Analysis by Treatment Method, 2023 to 2033
Figure 33: North America Market Y-o-Y Growth (%) Projections by Treatment Method, 2023 to 2033
Figure 34: North America Market Value (US$ Million) Analysis by Distribution Channel, 2018 to 2033
Figure 35: North America Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 36: North America Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 37: North America Market Attractiveness by Diagnosis Method, 2023 to 2033
Figure 38: North America Market Attractiveness by Treatment Method, 2023 to 2033
Figure 39: North America Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 40: North America Market Attractiveness by Country, 2023 to 2033
Figure 41: Latin America Market Value (US$ Million) by Diagnosis Method, 2023 to 2033
Figure 42: Latin America Market Value (US$ Million) by Treatment Method, 2023 to 2033
Figure 43: Latin America Market Value (US$ Million) by Distribution Channel, 2023 to 2033
Figure 44: Latin America Market Value (US$ Million) by Country, 2023 to 2033
Figure 45: Latin America Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 46: Latin America Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 47: Latin America Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 48: Latin America Market Value (US$ Million) Analysis by Diagnosis Method, 2018 to 2033
Figure 49: Latin America Market Value Share (%) and BPS Analysis by Diagnosis Method, 2023 to 2033
Figure 50: Latin America Market Y-o-Y Growth (%) Projections by Diagnosis Method, 2023 to 2033
Figure 51: Latin America Market Value (US$ Million) Analysis by Treatment Method, 2018 to 2033
Figure 52: Latin America Market Value Share (%) and BPS Analysis by Treatment Method, 2023 to 2033
Figure 53: Latin America Market Y-o-Y Growth (%) Projections by Treatment Method, 2023 to 2033
Figure 54: Latin America Market Value (US$ Million) Analysis by Distribution Channel, 2018 to 2033
Figure 55: Latin America Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 56: Latin America Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 57: Latin America Market Attractiveness by Diagnosis Method, 2023 to 2033
Figure 58: Latin America Market Attractiveness by Treatment Method, 2023 to 2033
Figure 59: Latin America Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 60: Latin America Market Attractiveness by Country, 2023 to 2033
Figure 61: Europe Market Value (US$ Million) by Diagnosis Method, 2023 to 2033
Figure 62: Europe Market Value (US$ Million) by Treatment Method, 2023 to 2033
Figure 63: Europe Market Value (US$ Million) by Distribution Channel, 2023 to 2033
Figure 64: Europe Market Value (US$ Million) by Country, 2023 to 2033
Figure 65: Europe Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 66: Europe Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 67: Europe Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 68: Europe Market Value (US$ Million) Analysis by Diagnosis Method, 2018 to 2033
Figure 69: Europe Market Value Share (%) and BPS Analysis by Diagnosis Method, 2023 to 2033
Figure 70: Europe Market Y-o-Y Growth (%) Projections by Diagnosis Method, 2023 to 2033
Figure 71: Europe Market Value (US$ Million) Analysis by Treatment Method, 2018 to 2033
Figure 72: Europe Market Value Share (%) and BPS Analysis by Treatment Method, 2023 to 2033
Figure 73: Europe Market Y-o-Y Growth (%) Projections by Treatment Method, 2023 to 2033
Figure 74: Europe Market Value (US$ Million) Analysis by Distribution Channel, 2018 to 2033
Figure 75: Europe Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 76: Europe Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 77: Europe Market Attractiveness by Diagnosis Method, 2023 to 2033
Figure 78: Europe Market Attractiveness by Treatment Method, 2023 to 2033
Figure 79: Europe Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 80: Europe Market Attractiveness by Country, 2023 to 2033
Figure 81: South Asia Market Value (US$ Million) by Diagnosis Method, 2023 to 2033
Figure 82: South Asia Market Value (US$ Million) by Treatment Method, 2023 to 2033
Figure 83: South Asia Market Value (US$ Million) by Distribution Channel, 2023 to 2033
Figure 84: South Asia Market Value (US$ Million) by Country, 2023 to 2033
Figure 85: South Asia Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 86: South Asia Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 87: South Asia Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 88: South Asia Market Value (US$ Million) Analysis by Diagnosis Method, 2018 to 2033
Figure 89: South Asia Market Value Share (%) and BPS Analysis by Diagnosis Method, 2023 to 2033
Figure 90: South Asia Market Y-o-Y Growth (%) Projections by Diagnosis Method, 2023 to 2033
Figure 91: South Asia Market Value (US$ Million) Analysis by Treatment Method, 2018 to 2033
Figure 92: South Asia Market Value Share (%) and BPS Analysis by Treatment Method, 2023 to 2033
Figure 93: South Asia Market Y-o-Y Growth (%) Projections by Treatment Method, 2023 to 2033
Figure 94: South Asia Market Value (US$ Million) Analysis by Distribution Channel, 2018 to 2033
Figure 95: South Asia Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 96: South Asia Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 97: South Asia Market Attractiveness by Diagnosis Method, 2023 to 2033
Figure 98: South Asia Market Attractiveness by Treatment Method, 2023 to 2033
Figure 99: South Asia Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 100: South Asia Market Attractiveness by Country, 2023 to 2033
Figure 101: East Asia Market Value (US$ Million) by Diagnosis Method, 2023 to 2033
Figure 102: East Asia Market Value (US$ Million) by Treatment Method, 2023 to 2033
Figure 103: East Asia Market Value (US$ Million) by Distribution Channel, 2023 to 2033
Figure 104: East Asia Market Value (US$ Million) by Country, 2023 to 2033
Figure 105: East Asia Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 106: East Asia Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 107: East Asia Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 108: East Asia Market Value (US$ Million) Analysis by Diagnosis Method, 2018 to 2033
Figure 109: East Asia Market Value Share (%) and BPS Analysis by Diagnosis Method, 2023 to 2033
Figure 110: East Asia Market Y-o-Y Growth (%) Projections by Diagnosis Method, 2023 to 2033
Figure 111: East Asia Market Value (US$ Million) Analysis by Treatment Method, 2018 to 2033
Figure 112: East Asia Market Value Share (%) and BPS Analysis by Treatment Method, 2023 to 2033
Figure 113: East Asia Market Y-o-Y Growth (%) Projections by Treatment Method, 2023 to 2033
Figure 114: East Asia Market Value (US$ Million) Analysis by Distribution Channel, 2018 to 2033
Figure 115: East Asia Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 116: East Asia Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 117: East Asia Market Attractiveness by Diagnosis Method, 2023 to 2033
Figure 118: East Asia Market Attractiveness by Treatment Method, 2023 to 2033
Figure 119: East Asia Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 120: East Asia Market Attractiveness by Country, 2023 to 2033
Figure 121: Oceania Market Value (US$ Million) by Diagnosis Method, 2023 to 2033
Figure 122: Oceania Market Value (US$ Million) by Treatment Method, 2023 to 2033
Figure 123: Oceania Market Value (US$ Million) by Distribution Channel, 2023 to 2033
Figure 124: Oceania Market Value (US$ Million) by Country, 2023 to 2033
Figure 125: Oceania Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 126: Oceania Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 127: Oceania Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 128: Oceania Market Value (US$ Million) Analysis by Diagnosis Method, 2018 to 2033
Figure 129: Oceania Market Value Share (%) and BPS Analysis by Diagnosis Method, 2023 to 2033
Figure 130: Oceania Market Y-o-Y Growth (%) Projections by Diagnosis Method, 2023 to 2033
Figure 131: Oceania Market Value (US$ Million) Analysis by Treatment Method, 2018 to 2033
Figure 132: Oceania Market Value Share (%) and BPS Analysis by Treatment Method, 2023 to 2033
Figure 133: Oceania Market Y-o-Y Growth (%) Projections by Treatment Method, 2023 to 2033
Figure 134: Oceania Market Value (US$ Million) Analysis by Distribution Channel, 2018 to 2033
Figure 135: Oceania Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 136: Oceania Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 137: Oceania Market Attractiveness by Diagnosis Method, 2023 to 2033
Figure 138: Oceania Market Attractiveness by Treatment Method, 2023 to 2033
Figure 139: Oceania Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 140: Oceania Market Attractiveness by Country, 2023 to 2033
Figure 141: MEA Market Value (US$ Million) by Diagnosis Method, 2023 to 2033
Figure 142: MEA Market Value (US$ Million) by Treatment Method, 2023 to 2033
Figure 143: MEA Market Value (US$ Million) by Distribution Channel, 2023 to 2033
Figure 144: MEA Market Value (US$ Million) by Country, 2023 to 2033
Figure 145: MEA Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 146: MEA Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 147: MEA Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 148: MEA Market Value (US$ Million) Analysis by Diagnosis Method, 2018 to 2033
Figure 149: MEA Market Value Share (%) and BPS Analysis by Diagnosis Method, 2023 to 2033
Figure 150: MEA Market Y-o-Y Growth (%) Projections by Diagnosis Method, 2023 to 2033
Figure 151: MEA Market Value (US$ Million) Analysis by Treatment Method, 2018 to 2033
Figure 152: MEA Market Value Share (%) and BPS Analysis by Treatment Method, 2023 to 2033
Figure 153: MEA Market Y-o-Y Growth (%) Projections by Treatment Method, 2023 to 2033
Figure 154: MEA Market Value (US$ Million) Analysis by Distribution Channel, 2018 to 2033
Figure 155: MEA Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 156: MEA Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 157: MEA Market Attractiveness by Diagnosis Method, 2023 to 2033
Figure 158: MEA Market Attractiveness by Treatment Method, 2023 to 2033
Figure 159: MEA Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 160: MEA Market Attractiveness by Country, 2023 to 2033






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Congenital Adrenal Hyperplasia Treatment Market Analysis and Forecast by Type, Treatment, End User, and Region through 2035
Crisis Management Market Size and Share Forecast Outlook 2025 to 2035
Cyber Crisis Management Market by Type, Application, Vertical, and Region-Forecast through 2035
Airline Crisis Management Software Market Size and Share Forecast Outlook 2025 to 2035
Tax Management Market Size and Share Forecast Outlook 2025 to 2035
Key Management as a Service Market
Cash Management Supplies Packaging Market Size and Share Forecast Outlook 2025 to 2035
Fuel Management Software Market Size and Share Forecast Outlook 2025 to 2035
Risk Management Market Size and Share Forecast Outlook 2025 to 2035
SBOM Management and Software Supply Chain Compliance Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Case Management Software (CMS) Market Size and Share Forecast Outlook 2025 to 2035
Farm Management Software Market Size and Share Forecast Outlook 2025 to 2035
Lead Management Market Size and Share Forecast Outlook 2025 to 2035
Pain Management Devices Market Growth - Trends & Forecast 2025 to 2035
Data Management Platforms Market Analysis and Forecast 2025 to 2035, By Type, End User, and Region
Cash Management Services Market – Trends & Forecast 2025 to 2035
CAPA Management (Corrective Action / Preventive Action) Market
Exam Management Software Market
Labor Management System In Retail Market Size and Share Forecast Outlook 2025 to 2035
Waste Management Carbon Credit Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA